Psychology - Structure of ice #part1

source
Today i will be discussing about the topic ''Structure of ice'', read through and share you thoughts, ideas in the comment section.
Table of contents
- Significance of the structure of water
- Behavior and properties
- Physical properties
- Conclusion
Significance of the structure of water
Water being in it's liquid state has a complex structure, this undoubtedly consists of considerable connection of active molecules. The production of larger values of viscosity, boiling point and surface tensions is made available solely with the help of extensive hydrogen. For instance, looking at the size of molecules, it is expected for water to have a boiling point at a degree of 100 °C (360 °F), and it is expected to be lower than the boiling point. In the condensed state of water (Liquid or solid) it is expected of the water to to exhibit extensive connection between the water molecules.
Looking at the popularity of water, the molecule in it plays a major role in disintegration of ionic compounds, in the process of formatting aqueous solution. There is a vast amount of dissolved salt in the oceans on earth, this dissolved salt found on the ocean provides a great natural resource. Additionally, the hundreds and thousands of chemical reactions that arise swiftly this is to keep the alive and take active place in aqueous filed.
Nevertheless, by the solubility of water of substance such as salt and sugar makes our food to be well spicy and flavored. The connection between the solute and the polar water molecules, for instance, substance being dissolved or disintegrated, plays an active crucial role. When the positive ends of water molecules infatuated by the anions, this is as a result of the dissolvation of the ionic solid, thereby making the anions positive by the attraction of molecules and cations negative. The process of changing this molecule positive and negative is know as hydration. This process called hydration tends to make the salt break and dissolve in water, in this process the hard forces present between the negative and positive ions of the solid are replaced with the water-ion connections.
This is an experiment of a dissolving candy M&M's
Ionic substance tend to break apart when dissolved in water, the division is usually split into cations and anions. For example, when sodium chloride dissolves in water the end solution is separated into Na+ and Cl− ions, as shown in the formula below;
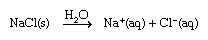
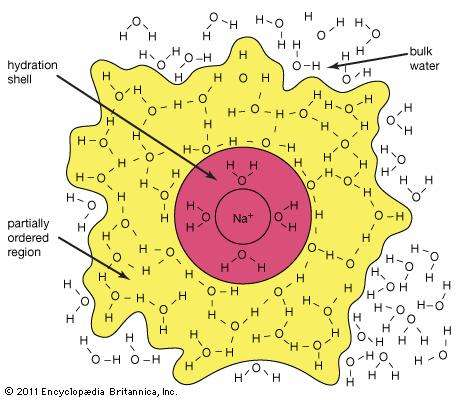
The (S) in the above equation represents the solid state, the (aq) on the other hand is an abbreviation for aqueous, this indicates that the ions are hydrated - this means there is an exact quantity of water particles attached to them - below is a diagram of a sodium ion.
source
Generally, the greater the charge of density, (this connotes a ratio of charge to surface area) of an ion, with the large hydration number. Negative ions as an active rule has a smaller hydration number than positive ions this is because the large number of of the crowding ions that takes place when the hydrogen atoms of the molecules are oriented toward the anion.
Having explained the significance of the structure of ice, there are many substance for which a water is declined as an acceptable solvent. Let's take for example an animal fat, is insoluble in water this is because the non-popular nature of water and fat molecules portrays them in a discordant with the molecules of a polar water. Generally, ionic and polar substance are soluble in water.
We shall the looking at the behavior and properties of ice in the next post.
In case you missed others, you can check them below
Psychology: How to tackle burnout #part 1
Turning without a differential (how does the train do it?)
Digital twins: where are we presently part #1
Digital twins: Where are we now part#2
Ischemic heart disease- one of the deadliest diseases in the world part #1
Psychology: How to tackle burnout part #2
Refrence :
(https://www.britannica.com/science/water/Structures-of-ice)
Join with us on Discord! https://discord.gg/taNc9Qr
The article is informative..
Water the essential solvent of live..
na yg silap rakan bg neu tuleh gamba jeut keu dabel....asoe tulisan bereh 👍
Terimakasih atas sarannya bang . Udah saya perbaiki .hehe
ok sm2 dek, alumni polyek syit nyoeh....lon dengeu2 berita
Thanks a lot bro
This post has received votes totaling more than $50.00 from the following pay for vote services:
minnowbooster upvote in the amount of $119.19 STU, $235.99 USD.
For a total calculated value of $119 STU, $236 USD before curation, with approx. $30 USD curation being earned by the paid voters.
This information is being presented in the interest of transparency on our platform @yandot and is by no means a judgement of your work.
Awedome article. thanks
I think its not water boiled point, 100 °C its corrected for raw water and demineralized water, after boilted the water change to steem and going to saturated steem.
Thanks for your correct brother . Minor mistake . I am already edit it . Thanks a lot!
You're welcome brother...can't wait to the next session part#2, I think its talk about correlation with psychology of human being.. Nice post form an motivation man.
Hi
Good post
I'm wondering, why use psychology as your first tag instead of science?
I think science is better as first tag
Cations, anions and water... You did a good job making these for easy understanding... Thanks
Great article
interesante articulo