A Revolution from an Accident, Blackbody Radiation and the Beginning of Quantum of Mechanics
Heart and Brain are the two lords of life. In the metaphors of ordinary speech and in the stricter language of science, we use these terms to indicate two central powers, from which all motives radiate, to which all influences converge.
Sometimes it happens that you search for answer of a puzzle and you come up with some kind of answer just to fit the solution in which you don't completely believe in. All on a sudden this starts a revolution and opens up so many doors which you couldn't see when you solved the puzzle. Planck's triumph to solve the puzzle for Blackbody radiation is such an example. In fact, the history of physics is full of such kinds of examples. Heinrich Hertz invented the radio wave and showed that light is an electromagnetic wave that passes without a medium. So, everything was settled. Light is an electromagnetic wave. In the end every scientist thought that they have answered the question which lasted for centuries. Lets move on.
But no. It just lasted for a few decades. But when the question was asked about radiation of matter and its origin, the wave theory of light couldn't completely answer it. So, again question was asked about light and its nature.Blackbody radiation
What is blackbody radiation? Before answering this question let us go through one example which we know from our daily life. Have you ever looked at a glowing metal? Its color goes from red to yellow all the way to white as it becomes hotter and hotter. Which color you will see depends on the temperature. Now, the question is does it radiates light when it is not hot enough? Well, yes it does. But most of it is in infrared. That is the reason we can't see them.
An object doesn't have to be hot to radiate Electromagnetic radiation. Objects radiate continuously. The question is only in which frequency?
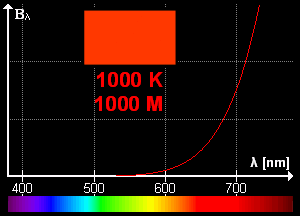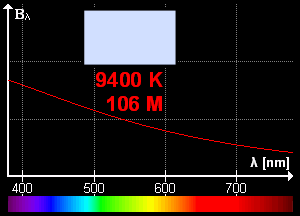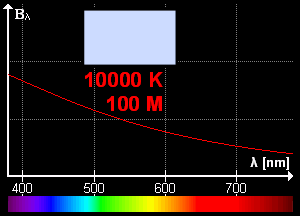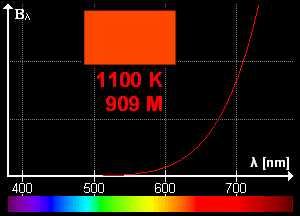
Color change with temperature | source
To be in thermal equilibrium with its surrounding the body has to radiate the same amount as he will absorb. If we can think of a body which absorbs all the energy falls on it and it doesn't reflect or transmit any part of the energy, then we can call it a black body. In nature balckbodies doesn't exist. It is just an idealization. One can ask why do we need this idealization. The answer will be that we can disregard whatever it radiates, because all the blackbodies behave the same.
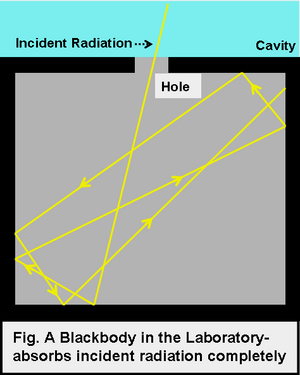
In laboratory, we approximate blackbody by cavity with a small hole. The radiation enters through the hole inside the cavity. Then reflected back and forth on the walls until it gets absorbed. The cavity walls are constantly absorbing and radiating energy. This is the property which we are interested in. This property is known as balackbody radiation. A blackbody radiates more when it is hot and less when it is cold. The spectrum of a hot blackbody has its peak in higher frequency than the spectrum in a cooler one.
An example can be when we heat an iron bar. As it gets hotter and hotter the color changes from red to yellow all the way to white. Now, what are the properties of blackbody radiation. Does it follow some laws or rules?
Properties of blackbody radiation
During the end of 19th century physicists were wondering about how objects glow. For example, our sun or lava. Is there any law by which they can know at which temperature it will glow and in which color? How much energy it will radiate in a certain temperature? To answer this question Lord Rayleigh and his colleague James Jean assumed a body which only absorbs or radiates and named it as balackbody.

They constructed a formula to explain the amount of radiance in a certain frequency, which we know today as Rayleigh-Jeans law.
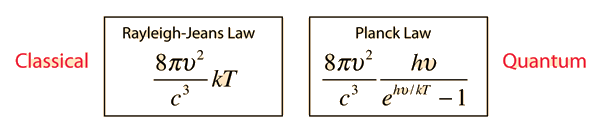
If we look at the figure, the first equation is Rayleigh-Jean's law. Here we can see that if the frequency increases, the radiance will increase a lot . Even it can goes to infinity. But, it will go against the law of conservation of energy. So, nothing can radiate an infinite amount of energy. This problem starts happening when we go towards the higher frequency like in ultra-violate ray. But it agrees with low frequency level. This problem is known as ultra-violate catastrophe. To solve this problem a German physicist came up with a mathematical model in which he was not so sure of. The name of this famous physicist was Max Plank. He proposed that when a blackbody radiates, it radiates in discrete energy (E = hν) which he called quanta. It depends on frequency. The next equation in the figure above is Plank's formula for blackbody radiation and it fits the experimental data well. So, the problem of ultra-violate catastrophe is solved. From Plank's equation we can approximate to Rayleigh-Jeans formula. But, this idea of quanta given by Plank has changed our whole idea of physics in microscopic world.
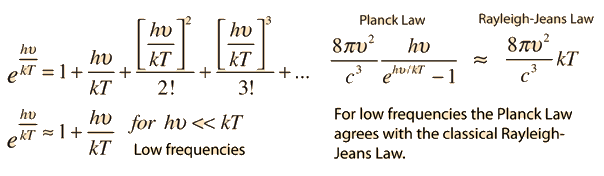
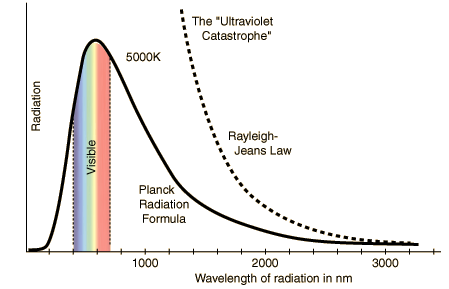
We can also see here how Rayleigh-Jeans law predicts that the radiance is going to infinity but Plank's formula predicts exactly how it is in experimental data.Physicist Wien came up with another law to describe the relation between the wavelength of the radiation and the temperature.
He said--
The wavelength, at which an object radiates most of its light, is inversely proportional to its temperature.
This means, the hotter the object is, the lower the wavelength it has. So, if a star is so hot, it will radiate in low wavelength or high frequency. And it will look like blue. If it has low temperature, it will radiate at high wavelength. So, the star will look reddish.
Photoelectric effect
Until Photoelectric effect was invented scientists thought of light as a wave phenomena. So, what is photoelectric effect? When light shines on a material, it emits electron. What happens here is that when light falls on material it excites the atoms in the material, which emit electrons. But, not light of every frequency does it. So if light is wave, then it would happen for every frequency. Einstein was wondered by this phenomenon. To solve this problem he went to Planck;s approximation for blackbody radiation which says that light is discrete energy( E= hv ). We know it as photon. This is how he solved the problem which brought him the Nobel prize.
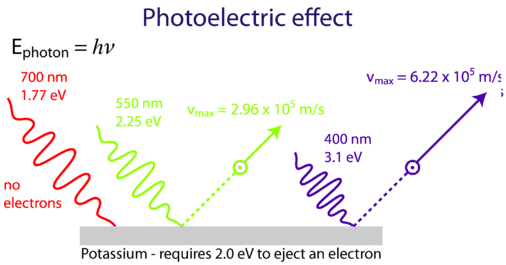
Plank's mathematical model predicts that light is like discrete energy, in which he completely didn't believe in. But, this idea solved the photo electric effect problem and as well as it gave light a new definition. This definition later opened the door for a new theory of physics, which we know as the quantum theory.
References
[1] Kristen Rolfs , Tools of Radio astronomy
[2]Young and Freedman,University Physics
[3]Olga Atanackovic, Opsta Astrofizika
[4] Dejan Urosevic, Theoriske osnovne Radio Astronomija
[5] https://en.wikipedia.org/wiki/Black-body_radiation

Thank you very much for being here and reading my post, I really appreciate your support and comments.
Until next time,
have a nice time and don't forget to steem on

Haha, sorry I cant read your article. I'm just reading "the quantum universe: everything that can happen does happen" by brianthe cox & jeff forshaw. I dont want "spoilers" 😅 but i'm very happy to find articles like these on steemit!
ha ha ha . that is an amazing book. i hope that you will enjoy your time :D
Yea I love it! I'm only one fifth in, already dreamt of quantum physics twice :) bookmarking your article for a later read though.
Hi! I really like your post. I'm a chemistry student, and the blackbody problem was one of my favorite parts of my modern physics course.
I think that the beauty of the planck's conclusion is that it worked so well with thath of other scientist, as Boltzmann, Einstein and later Bohr.
The quantic world is just exciting! Steem On!
Very interesting post. You explained everything so nicely.
thank you ana very much.
Thank you @rifkan for this article! I am a chemistry and biochemistry student so I have to spend quite some time studying physical chemistry. Not a big fan to be honest but this is a very easy to understand introduction into electromagnetic radiation! Are you going to write something about quantum mechanics - like tunneling?
I am trying to get people more engaged with science and I would love some input from other scientists. I would greatly appreciate if you could look a my posts and share your opinion. This is not asking for upvotes, but I want to write good informative, easy to understand blogs about science, and since you did a great job here, I would love your input! Thank you again. Cheers!
well-written. I like the way you explain it. Thanks man!
hello, good post! I invite you to stop by my blog so you can see the recipes I publish daily, I hope you like them, greetings!
Nice explanation
Congratulations @rifkan, this post is the most rewarded post (based on pending payouts) in the last 12 hours written by a Newbie account holder (accounts that hold between 0.01 and 0.1 Mega Vests). The total number of posts by newbie account holders during this period was 4172 and the total pending payments to posts in this category was $2429.45. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Congratulations, your post received one of the top 10 most powerful upvotes in the last 12 hours. You received an upvote from @hendrikdegrote valued at 156.70 SBD, based on the pending payout at the time the data was extracted.
If you do not wish to receive these messages in future, reply with the word "stop".
So, Am I the only one that actually played with my screen for a few minutes there??😂😂😂