Carbon and Earth: A Story of Life

The story of earth and carbon goes back billions of years. This is an epic tale, one pieced together from clues buried in the shadows of history. In those shadows are secrets that can tell us how earth developed into a planet that supports life. Carbon is at the heart of this miracle. How did earth come by this gift? And what role does carbon play in the life of our planet?

In the Beginning...
As new clues appear, different versions of earth's history are offered. One version describes a collision that occurred more than 4 billion years ago. According to this concept, an embryonic, "Mercury-like" planet crashed into earth. For this scenario to be viable, the small planet would have needed certain characteristics. Its core had to be made of iron--and another element, possibly silicon. Its crust had to be rich in carbon. At impact, the core of the small planet is supposed to have sunk into the core of the earth, and its crust to have melded with earth's crust.
Voilà! With the extinction of an errant, embryonic planet, earth gained the gift of carbon.

Mercury, as shown in the picture, appears dark because of significant amounts of carbon in its crust. The presence of carbon was discovered in 2015, when space probe Messenger crashed into the planet and took samples of the surface.
Another version of earth's carbon history centers around a bombardment of comets that may have lasted for more than a billion years. This relentless barrage of extra-terrestrial bodies is often called the Late Heavy Bombardment. It is suggested that water and carbon may have been delivered to earth during the bombardment.

Cyanobacteria Oxygenate the Atmosphere
Whatever the chronology of events in earth's early years, at some point carbon arrived, the planet began to cool, and the environment became amicable to the development of a simple life form: cyanobacteria. According to scientists from the University of California (Berkeley), cyanobacteria are the oldest known fossils. The bacteria are sometimes called blue algae because they contain two chemicals that give them a blue green cast. These chemicals, chlorophyll and photocyanin, carry out photosynthesis. It was cyanobacteria's ability to perform photosynthesis--to pull carbon from the atmosphere and return oxygen--that made these microorganisms so vital to the evolution of life on earth.

*The dramatic increase in oxygen produced by cyanobacteria is estimated to have occurred a little less than 2.5 billion years ago. This dramatic change in earth's atmosphere is often referred to as the Great Oxidation Event*.
The Rise of Plants
Plants support life on earth, and cyanobacteria are credited with facilitating the rise of plants. The bacteria did this by inhabiting small cells called eukaryotes. When cyanobacteria and eukaryotes combined, (this is called endosymbiosis,) they both benefited. Eukaryotes were endowed with the ability to make their own food (because cyanobacteria were their guests) and cyanobacteria had a place to live, and receive nutrients.
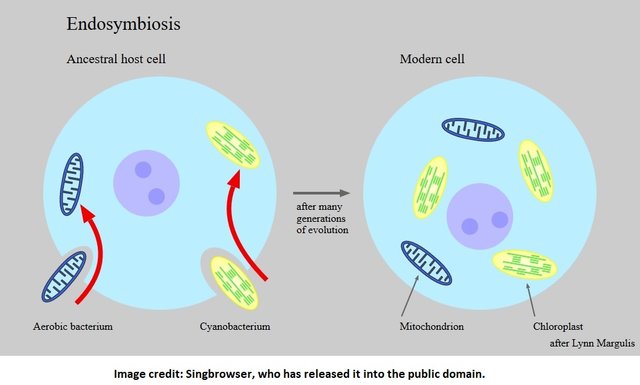
Eventually, plants grew larger. More carbon was removed from the atmosphere, and more oxygen was produced.
The gifts of cyanobacteria did not end there. An article in the ISME Journal (Multidisciplinary Journal of Microbial Ecology) describes how cyanobacteria became the ancestors of chloroplasts. Chloroplasts, of course, are the microscopic factories in which plants make food for themselves by transforming sunlight, water and carbon into glucose.
Chloroplasts are so essential to plant life, that scientists have so far only discovered one parasitic species of plant that is completely devoid of these plant factories.
Additional Benefits of Cyanobacteria
Ancient cyanobacteria were instrumental in the development of petroleum. Today, they are a food source for some people. They help to fix nitrogen in soil and thereby help to increase crop production. They are also used to bind soil in prairies, and therefore help to limit erosion.
Cyanobacteria can also be harmful. They create algal blooms that choke off oxygen and destroy marine life. Some types of cyanobacteria produce deadly toxins for which there is no antidote.
%20public.jpg)
Lake Debar, in Macedonia during an algal bloom. The lake was formed when a power plant was constructed in nearby Shpilje.
Rocks and Oceans Are Reservoirs for Carbon
This is a fascinating part of the carbon story, one that is not often considered by many people. As the earth matured, carbon was taken out of the atmosphere in different ways. One of these, already mentioned, was through photosynthesis. Another was through the 'weathering' of rocks.
Carbon dioxide in the atmosphere combines with moisture and becomes part of a cloud. When rain falls from the cloud, it is acidic (carbonic acid). Minerals in the rain combine with minerals in the rocks. Eventually the rocks dissolve, and are washed into the ocean by streams and rivers. Over thousands of years, some of the carbon captured by the rocks and washed into the ocean will return to the atmosphere. But some will fall to the bottom of the sea and be trapped there.
Rock Weathering
_(20189173003).jpg)
Rock disintegration and talus formation on Mount Sueffels, Colorado". The description and image are from a 1913 publication, A Treatise on Rocks, Rock Weathering, and Soil, by George Perkins Merrill. The picture is in the public domain.
Another important way carbon is removed from the atmosphere is through exposure to the ocean. Carbon dioxide is freely exchanged between the atmosphere and the ocean. However, environmental influences tend to keep more CO2 in the ocean than is returned to the atmosphere. The CO2 that remains in the ocean is "neutralized" at the bottom of the sea. This is a very, very slow process because carbon and oxygen do not easily break the bond between them. It would be helpful to the climate (scientists believe) if the process of neutralization could be sped up.
That is exactly what scientists from Caltech believe they have achieved. Researchers from the Resnick Institute discovered they could increase the rate at which oceans break down and store CO2 . What they learned was that by adding the enzyme carbonic anhydrase to sea water, they increased by a factor of 500 the rate at which CO2 was converted to carbonic acid (H2CO3 ). When this acid falls to the sea floor, the carbon is trapped.
Heat-trapping Mechanism of Carbon Dioxide
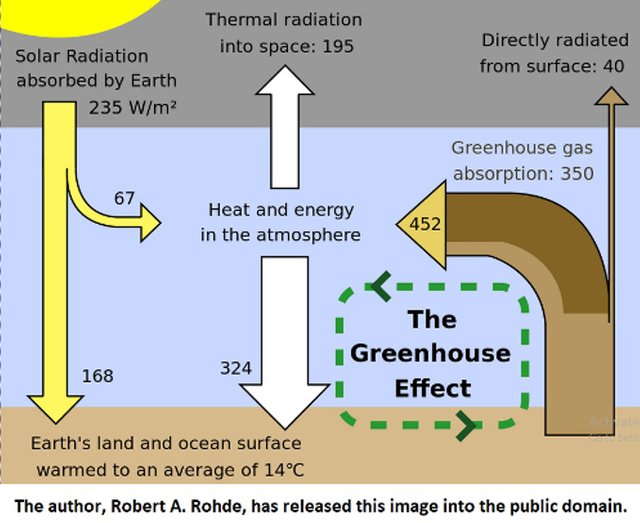
Carbon dioxide is implicated in global warming because it absorbs infrared light. Invisible light, sent by the sun to earth, passes through the atmosphere. Earth sends the light back in infrared waves, which mostly pass through the atmosphere, except for the small amount absorbed by water and carbon dioxide(and some other gases, including methane.) The infrared waves are then sent back to the earth. This has a warming effect. The more carbon dioxide present in the atmosphere, the greater the warming effect. Hence, the term Greenhouse Effect was coined to explain earth's rising temperatures related to increased carbon dioxide in the atmosphere.
The Carbon Cycle and Sequestration
We know carbon is essential to life on earth. And we know that the amount of carbon in the atmosphere affects the ability of life to thrive on this planet. We also know that there are several ways by which carbon is naturally removed from the atmosphere. What hasn't been discussed here yet, is how carbon gets into the atmosphere in the first place (besides human activity). If we look at Hawaii today, we have our answer. Carbon bilges out of the earth in volcanic eruptions and seeps out in gases associated with volcanoes. If it weren't for natural methods of carbon trapping described here, carbon would build up in the atmosphere to levels that would not be amenable to life.
But those mechanisms do exist, and they have worked pretty well at keeping carbon production and extraction in balance. Up until now, that is. This is where the debate about fossil fuels, global warming and carbon sequestration comes in.

The caption under this picture identifies the children as 'Breaker Boys' at the Woodward coal mine in Pennsylvania. The children were employed to sort and grade coal outside the mine. The picture is dated 1900 and is in the public domain because its copyright has expired.
Locked in the earth's crust is carbon. The carbon exists in many forms. Two of these are well-known to us--petroleum and coal. Tapping the crust of the earth to extract and burn these fuels introduces vast amounts of carbon into the atmosphere, carbon that cannot be captured, naturally, as quickly as it is produced. When environmentalists talk about trapping and sequestering carbon, this is the imbalance they are trying to correct. If we are going to introduce carbon into the atmosphere at a rate faster than nature can remove it, then perhaps we should find a way to give nature an assist, through trapping and sequestering. Either that, or we should stop upsetting the carbon balance. That's a simplified version of the environmentalist's argument.
Carbon Dioxide Builds Over Yellowstone Volcano
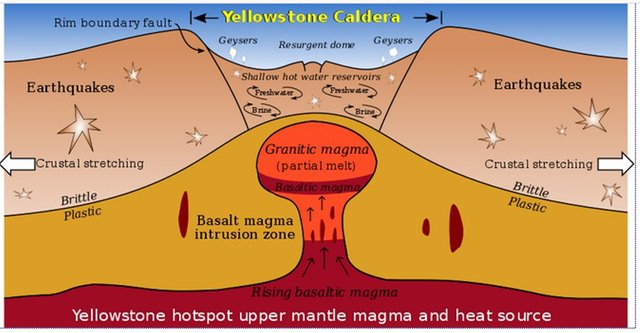
This is a diagram of the Yellowstone Caldera and magma plume. The picture was released into the public domain by its author, Kbh3rd.
In 2016, it was reported that high concentrations of CO2 were reported over Yellowstone Volcano. In 2017, it was reported that Yellowstone's super volcano was experiencing an "earthquake swarm". It's not known if these two factors together--the carbon dioxide buildup and the earthquake swarm--suggest an immanent eruption. If the volcano does erupt, enormous amounts of CO2 will spew into the atmosphere.
Carbon
Since the star of this story is carbon, it merits some definition. The chemical symbol for carbon is C. It is the sixth element on the periodic table. There are six protons and six neutrons in its nucleus, six electons in its rings. The charm of the carbon atom, the secret to its utility and success as a life-giver, is its tendency to combine with other elements, and its ability to form long chains.
According to LiveScience, about ten million carbon compounds have been discovered to date. As a matter of fact, carbon forms more compounds (combines with other elements) than any other element on earth, despite the fact that there are elements much more abundant on the planet.
CO2: Carbon is solid and oxygen is a gas at room temperate. When they connect, they share electrons, which creates a strong bond. The gas CO2 makes up about .04% of the earth's atmosphere and oxygen makes up about 21%.
The name carbon was coined by Antoine Lavoisier in the later half of the eighteenth century. However, carbon, in the form of coal, was discovered by early humans.
Notes
Although carbon seems to be the most likely basis for life--because of its rich chemistry--it is conceivable that life may exist somewhere else with another chemistry as its basis, silicon or arsenic perhaps. We are cautioned by Stephen Hawking, and other scientists, to think beyond the familiar and expected when contemplating the possibility that life may exist without carbon.
Acid buildup in oceans is a significant environmental concern not touched on here. Oceans absorb carbon dioxide, but not without consequence to marine life. This is a broad discussion, for another blog.
Besides rocks and oceans, there are other important carbon reservoirs. These include, but are not limited to, soil and plants, especially large woody plants.
Greenhouse gases, besides CO2, are produced by human activity. One of the most important is methane. There are others that have varying effects on climate.

The Amazon Forest in Brazil. The Amazon is a carbon sink--a natural reservoir for carbon. Picture source: Pixabay
Thanks for a great and informative post, I really enjoyed it and learned quite a few things. I never knew Mercury had that much carbon, for instance. Interesting.
One thing I did know was the connection between carbon and life, and the possibility of non-carbon-based life forms, which is very interesting... Another substance tightly connected to life on earth is water, and especially it's component oxygen. I have the impression that the same thing could be said about that, that there might be life forms elsewhere breathing and drinking other things. I dream of life forms on Saturn's moon Titan, based on the methane circling the three forms as water does on earth. At least I can dream... :)
New ideas come from dreams... I wake up in the morning with some of my most inspired insights. Thanks for your comments. I learned a great deal in writing this, so my mind is open to possibilities. Many mysteries surrounding the origins of life on earth... many more surrounding the possibility of life elsewhere.
True. The possibilities seem to be infinite
Great research! Deep work! I am not very good reading in English, especially scientific articles with specific terminology, so it will take me some time to get the bottom)) And while I'm still in reading, I already want to say thank you for making my conscious wider!
Thank you. It's hard reading in a language that is not your own. I struggle in Spanish and German, so I understand the difficulty. Thanks for stopping by.
You won't believe it but I was just looking at th3 next page on my dictionary, looking for which word to illustrate on it. And carbon popped out at me and somehow I knew I wanted to draw it. I usually research my words so I have a basis for my drawing and this article is just perfect!
Isn't it amazing how all life and the earth itself exists because of this one element?
Yes. This blog began with a question about grass. I followed the story back and ended up here. An amazing story. Look forward to reading your blog.
That ecxelente post too interesting, uff how many things to learn. I only know that all this perfection and synchronization of elements for the life of the planet must go beyond materiality. But it is extremely important and pleasant to know that there are people who are interested in knowing about the origin of existence on this earthly plane. I personally believe that there is an original source that created everything perfectly.
Thank you for your kind comment. I hope that by using simple language and explaining the issue in simple terms, some people might become interested. I learned a lot myself in writing this blog. It was like going back to school.
Caves and cave formations are a significant carbon sink by sequestration of CaCO3.
Thank you! There was so much to say, so much to explain. The more I read, the more I discovered. I could have done a blog on rock weathering alone. Or on cyanobacteria. Or on carbon's inclination to bond. I'll look up those cave sinks now :)
And further down the carbon rabbit hole you go!
:))
You received a 60.0% upvote since you are a member of geopolis and wrote in the category of "geopolis".
To read more about us and what we do, click here.
https://steemit.com/geopolis/@geopolis/geopolis-the-community-for-global-sciences-update-4
Thank you!
I thought the 'faces of coal' might be a movie exaggeration, but those kids look exactly like they would in a movie based on a Dickens novel.
I know. I was looking for pictures of mines, but when I saw the kids, I had to put it up. No words.
holy wow... thank you for this amazing sharing... x (((o)))
:) Thanks for stopping by.
This is really a very good science post, I think you may have been quite unlucky and gone unnoticed on this one.
You don't know how good that makes me feel. Without objective feedback, there's no way to know if I've hit the mark or not. Respect your opinion highly. So... on I go to the next challenge :)
You probably could have broken this into two posts, or even made it part of a larger series. A lot of the most experienced authors on steemstem do that. It's good for the readers, and you don't have to start the research process from scratch for each post
You're right. I was fascinated with carbon--the subject made sense to me as I researched in a way it hadn't before, so I presented the whole picture. But I can see more posts growing out of this. I appreciate the input.