Body Pigments [Part 3: Hemoglobin, Myoglobin and Bilirubin]
Have you ever wondered why you have certain structures in your body; hard structures like bones and circulating fluids like blood. You might have asked why people's eye color differ and why the blood decided to take up the color of ketch up and tomatoes. Well, the answer lies in the component these structures are made up of: Bone is hard because it contains calcium, eye color differ due to varying quantity of melanin and blood is red because it contains hemoglobin.
In today's post, we shall be studying the hemoglobin molecule along with myoglobin and other similar pigment molecules.
Image Source = Pixabay :: Licence = CC0 Creative Common
In previous posts, I introduced us to chemical compounds responsible for different colors exhibited by living organisms, one of which is melanin found in the skin. Melanin along with hemoglobin of red blood cells are responsible for the skin color of a person. These compounds are known as bio chromes or biological pigments. Although, they make the world colorful and distinguishable, this is not their primary function. Hemoglobin primary function is to transport oxygen from the lungs to all tissues of the body. Oxygen is essential in the process of producing energy needed for cellular activities. Not having hemoglobin in the blood, or rather having inactive hemoglobin has the same effect of not breathing.
What is Hemoglobin?
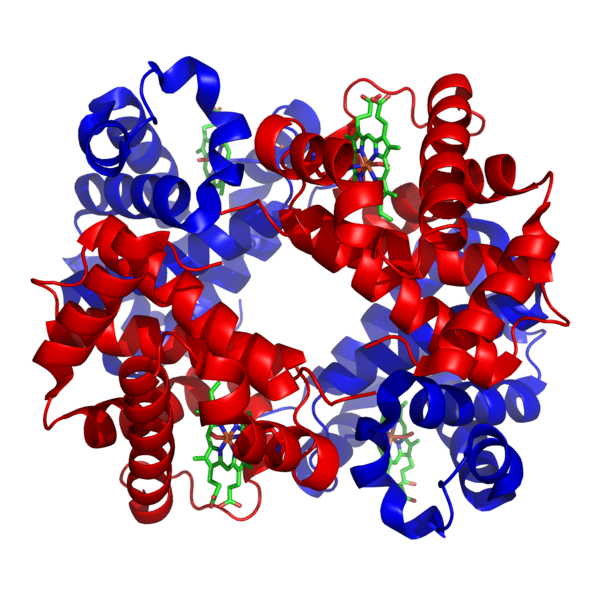
Red blood cells are made up of hemoglobin molecules
Author = Zephyris at English Wikipedia :: Licence = CC-BY-SA-3.0 :: Via Wikipedia Commons :: Unedited :: Image Source = Wikipedia Commons
Hemoglobin is a blood pigment responsible for the red coloration of blood, without it blood will be straw yellow in color. It is responsible for picking up oxygen molecules from the lungs as well as transporting CO2 molecules for excretion. In normal conditions, the quantity of hemoglobin in the blood of an adult male ranges between 14 - 16g/dL. In female, it is 13-15 g/dL and between 11 - 16 g/dL in children. This amount is kept constant majorly by the liver. Excess Hemoglobin in the blood can be traced to diseases such as polycythemia. Polycythemia is a condition whereby the body produces excess red blood cells. Patients suffering from polycythemia are higly susceptible to stroke and cardiac attack. Other reasons for high hemoglobin content of the blood can be traced to dehydration and smoking.
Abnormally low hemoglobin content is usually accompanied with jaundice(yellowing of eyes and skin). Hemoglobin is composed of Iron, Globin and Porphyrin. During the destruction of red blood cells in thin capillaries, hemoglobin get released into the blood. As the blood moves into the liver, macrophages degrade hemoglobin into iron, globin and porphyrin. The iron derived is stored for later use in a less reactive form called ferritin. Globin is also stored for later use. However, porphyrin is degraded into a yellow pigment molecule called bilirubin, this is necessary because porphyrin is highly reactive and dangerous when free in blood. Bilirubin is then excreted by the liver, but in conditions whereby there is an abundance of bilirubin, it begins to color the eyes and skin yellow- this condition is known as jaundice.
There are two major types of hemoglobin constituting the red blood cells. These are adult hemoglobin denoted by HbA and Fetal Hemoglobin denoted by HbF. There are two types of adult hemoglobin: HbA1 and HbA2. Fetal hemoglobin is found in abundance in blood of fetuses and babies. Replacement of this hemoglobin by adult hemoglobin initiates immediately the child is born. The difference between these three hemoglobin is in the arrangement of their molecular sub-units. Hemoglobin is a metalloprotein with the iron containing molecule called heme, conjugated with a protein called globin (NB: Heme contains Iron and prophyrin).
The iron in heme is present in ferrous form (Fe2+). The porphyrin part of heme is a pigment molecule formed by four pyrrole rings attached to one another by methane bridges. Globin is formed by four polypeptide chains. In every hemoglobin molecule, there are four heme residues of heme taking up the space of a subunit; deductively, there are four sub-units. In adult hemoglobin A1, globin contain two alpha chains and two beta chains. In fetal hemoglobin, it has two alpha chains and two gamma chains, while Adult hemoglobin A2 has two alpha delta chains. A normal adult blood contains 97% HbA1, 1% HbA2 and 1% HbF.
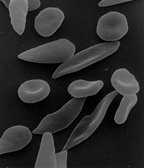
Sickle shaped red blood cells compared to normal shaped cells, this difference boils down to replacing glutamine with valine at the 6th position of amino acid sequence
Image Source = Wikipedia Commons :: Licence = Public Domain
Hemoglobin in the body has two major functions which includes transport of gases and buffer action. The two gases hemoglobin aim to transport are oxygen and carbon monoxide. A single hemoglobin molecule has the ability of binding with four molecules of oxygen. There are about 250 million molecules of hemoglobin forming a red blood cell, this equates that a single red blood cell transports about a billion oxygen molecules. Oxygen binds to hemoglobin by a physical reaction known as oxygenation to resulting information of a scarlet red molecule called oxyhemoglobin. Oxyhemoglobin is responsible for the bright colored blood that flows in arteries. When hemoglobin dissociates from oxygen, it picks up carbon monoxide by forming a compound referred to as carbhemoglobin. This molecule is purple-red and is responsible for dark coloration of venous blood, this is why a vein appears blue or purple from under the skin.
However, there is an odorless gas known as carbon monoxide produced by incomplete combustion of organic materials. This gas has high affinity for hemoglobin than oxygen and will compete with every oxygen for a hemoglobin to form carboxyhemoglobin. This gas is present in fumes of generator, cars as well as produced indoor by ovens and stoves.
It could be dangerous to health as to leading to death when inhaled in large quantity. There are indoor devices which help detect the presence of this gas since it can not be detected by the human olfactory system.
There are other compounds similar to hemoglobin
Hemoglobin is not the only oxygen transporting molecules in nature. A good example of a pigment molecule with affinity for oxygen in human body is myoglobin. Myoglobin is oxygen binding protein found inside of muscle cells. Unlike hemoglobin, it doesn't transport oxygen, it is more concerned with storage of oxygen allowing an organism hold its breath for a longer period of time. Myoglobin has a higher affinity for oxygen than hemoglobin, this is essential if it must perform its functions.
The amount of myoglobin in skeletal muscle is 2.5 g/100g of muscle, 1.4 g% of cardiac muscle and 0.3 g% of smooth muscle. Except in case of damage to muscle tissue, myoglobin is not present in blood. The process by which myoglobin is released into the blood is called rhabdomyolisis. This has a clinical importance as its an indicator of myocardiac infarction. Myoglobin in blood excreted by the kidney and could cause acute kidney injury.

The food industry uses the presence of myoglobin in meat to advantage in sales, they expose meats to carbon monoxide to make it pink red. This appearance is associated with freshness by consumers
Image Source = >Wikipedia Commons :: Licence = CC0
Another similar molecule to hemoglobin is Hemocyanin. Hemocyanin (Hc) unlike hemoglobin are not bound to blood cells, they exist in an open circulatory system of certain invertebrates such as the grasshoppers. Its metal is not iron but copper; when oxygen binds with it, it changes grom being colorless to being color blue. It is the most abundant oxygen binding pigment after hemoglobin in nature.
Other similar compounds like hemoglobin in nature include: Coboglobin, Vanabins, Erythrocruorin, Pinnaglobin, Lhegemoglobin, and Chlorocruorin.
The breakdown of Hemoglobin produces other prominent biochromes
A red blood cell as a living matter has an average life span of about 120 days after which it is destroyed. The senile red blood cell while passing through very thin capillaries, especially that of the spleen breaks to release its free hemoglobin into the blood. This hemoglobin is broken down in the liver into iron, porphyrin and globin. Iron and globin enter into a recycle pathway while the liver deals with excreting the dangerous compound- porphyrin.
The liver deals with porphyrin by first degrading it into a yellow pigment called Biliverdin. The bilirubin is then excreted along with bile into the small intestine, here about 50% of bilirubin is converted into urobilinogen by intestinal bacteria. Up to 5% of urobilinogen is excreted by the kidney in urine but as urobilin- this because urobilinogen when it comes in contact with air is converted to urobilin. Urobilin is responsible for the coloring of the urine.
The remaining 50% of conjugated bilirubin enters back into the blood to be excreted by the liver again, and the cycle continues till bilirubin leaves the body through urine or feces as stercobilinogen. Some urobilinogen don't diffuse back into the blood but are converted to stercobilinogen . Stercobilinnogen is responsible for the red coloration of feces.
Further Reading
Image Use Declaimer
All images used in this post are under Public Domain, CC0 and CC-BY-SA-3.0 licencing. Images needing attributions are attributed properly after immediately usage.


Go here https://steemit.com/@a-a-a to get your post resteemed to over 72,000 followers.