Liquid-Liquid Equilibria of (Water + Propionic Acid + Dimethyl Phthalate)
Liquid-liquid extraction is one of the separation methods used in the chemical industry. Reliable equilibrium data of the mixture to be separated for the design of the extraction devices and the success of the separation process are needed. For this purpose, previous studies on water-propionic acid-solvent systems have been investigated . Phthalic esters were found to give satisfactory results with acetic acid . Therefore, liquid-liquid equilibrium data generated with dilute aqueous solutions of dimethyl phthalate propionic acid were investigated.

The triple liquid-liquid equilibrium data of the water-propionic acid pair with dimethyl phthalate was experimentally determined at 298.15 K, 303.15 K and 308.15 K. Correlations of Othmer-Tobias and Hand were applied to test the reliability of experimental data. Distortion Factors (Di) and Separation Factors (S) were calculated by using the equilibrium data.
Used Chemicals
Propionic acid, CH3CH2COOH, was first described by J. GOTTLIEB in 1844. J.J Dumas found that the compounds included in the group known as fatty acids in 1848. Since it is the first fatty acid that can be separated by adding salt from aqueous solutions, it is also called propionic acid, meaning first oil in Latin . It is oily, scented, sour. Dimethyl phthalate, C10H10O4, is industrially produced from phthalic anhydride and methanol. Used in plasticizers and fly repellents for solvent and cellulose acetate and cellulose acetate-butyrate compositions . Some physical properties of the chemicals used are given in Table 1 [11].
| -------- | Propionic Acid | Dimethyl Phthalate |
|---|---|---|
| Mol Weight | 74,08 g/g-mol | 194,19 g/g-mol |
| Density | 990 kg/m³ | 1,19 g/cm³ |
| Boiling Point | 141,2 °C | 283,8 °C |
| Refractive Index [Literature] | n20/D 1,3869 | n20/D 1,5138 |
| Refractive Index [Experimental] | n20/D 1,38633 | n20/D 1,51688 |
Finding Liquid-Liquid Equilibrium Data
The coupling line for the water-propionic acid-dimethyl phthalate system was experimentally determined at 298.15 K, 303.15 K and 308.15 K. Ternary mixtures, estimated to be in the heterogeneous region and were mixed at constant temperature for 30 minutes in a ST 402 type shaker water bath. Each sample taken into centrifuge tubes was centrifuged at 6000 rpm for 5 minutes to separate the phases. Samples from each phase were analyzed on the HP 6890 Gas Chromatograph. Solvent and propionic acid in FID detector and water amount in TCD detector were determined.
| Column | HP Innowax Kapiler Kolon 19091N-213 |
|---|---|
| Dedector | Serial connected FID and TCD |
| Internal Standart | n-Propanol |
Dispersion Coefficient and Separation Factor
Dispersion coefficients of propionic acid and water in water-propionic and acid-dimethyl phthalate systems are percentages in solvent and water phases. The separation factors are the water-to-water ratio of the propionic acid distribution coefficient. Accordingly, distribution coefficients and separation factors are calculated from the equations (1) and (2) respectively.
(1) Dispersion Coefficient (Di): Di = Wi3 /Wi1
(2) Separation Factor (S): S= D2 / D1
Evaluation of Results
The Othmer-Tobias and Hand correlations were applied to test the reliability of the liquid-liquid equilibrium data obtained at 298.15 K, 303.15 K and 308.15 K of the water-propionic acid-dimethyl phthalate system. The equations for correlations are shown in equations (3) and (4) respectively.
(3) ln [(100-W33)/W33] = a1 + b1 ln [(100-W11)/W11)]
(4) ln (W23/W33) = a2 + b2 ln (W21/W11)]
- W33 : The amount of solvent in the solvent phase (as a percentage composition)
- W11: The amount of water in the water phase (as a percentage composition)
- a11 and b11: Othmer-Tobias correlation coefficients
- W23: The amount of propionic acid (as a percentage composition) in the solvent phase
- W21: The amount of propionic (in percent composition) in the water phase
- a2 and b2: Hand correlation coefficients
Results
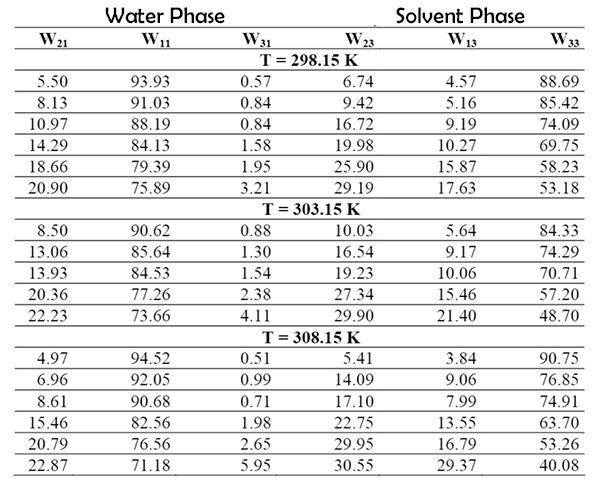
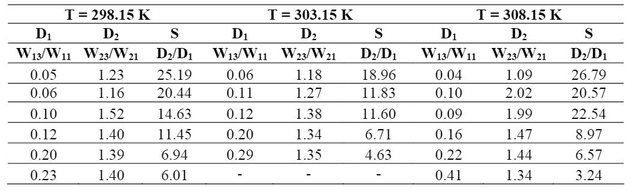
Experimental liquid-liquid equilibrium data of the water-propionic acid-dimethyl phthalate triple system are presented in Table 2. Table 3 shows the distribution coefficients and separation factors obtained at temperatures of 298.15 K, 303.15 K and 308.15 K, respectively.
In Figure 1 and Figure 2, the Othmer-Tobias and Hand correlations are shown graphically. The results obtained from correlations are given in Table 4.
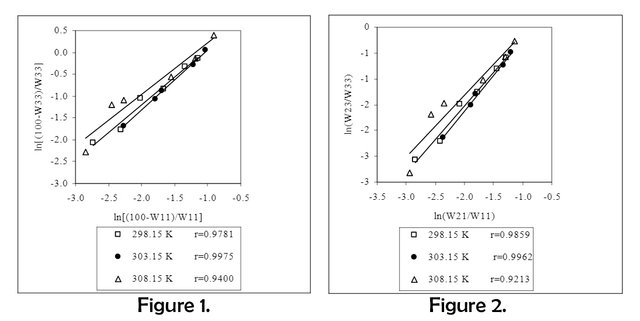
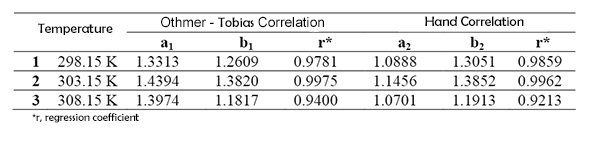
Correlation coefficients indicate that experimental data are reliable. The liquid-liquid equilibrium diagrams obtained for each temperature using experimental data are given in Figures 3-5.
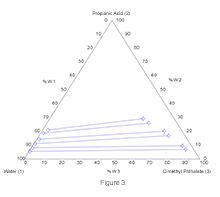
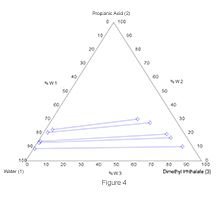
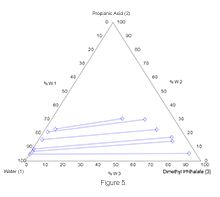
References:
- Arce, A., Blanco, A., Sauza, P. and Vidal, I., “Liquid-Liquid Equilibria of the Ternary Mixtures Water + Propanoic Acid + Methyl Ethyl Ketone and Water + Propanoic Acid + Methyl Propyl Ketone”, J. Chem. Eng. Data, 40, 225-229, 1995.
- Radwan, G.M., Al Muhtaseb, S.A., “Phase Equilibria of The Ternary System Water + Propionic Acid + 2-Butanol”, Separation Science and Technology, 32 (8), 1463-1476, 1997.
- Ullmann Encyclopedia of Industrial Chemistry, 5th ed., A5-225, A4-386, B3 (6-3), New York, 1993.
Congratulations @kedi, this post is the second most rewarded post (based on pending payouts) in the last 12 hours written by a Newbie account holder (accounts that hold between 0.01 and 0.1 Mega Vests). The total number of posts by newbie account holders during this period was 1095 and the total pending payments to posts in this category was $592.91. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Congratulations @kedi! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP