Diamonds in The Sky – Part 3: Diamond Oceans (Episode 2/2)
Oceans of liquid diamond surround the planetary core of Neptune.
Let's discover why.
In the first episode, we dived deep into Neptune’s atmosphere through clouds of methane, ice water and then sulfide compounds. We plunged deeper, into a sphere of water where diamond flakes danced in suspension around us. Inside the sphere of water, an ocean of diamond awaited us with large crystalline diamonds floating as icebergs at its surface.
In this episode, we will review the science behind this imaginary voyage. Diamond oceans roaming within the hearts of Uranus and Neptune? Yes, this idea is indeed highly probable. Let’s see why.

3/ Why do we believe that outer giant gas planets contain an ocean of diamond?
Our imaginary expedition was exciting, beautiful and truly stunning. But is there really an ocean of diamond surrounding the core of Neptune (and Uranus)? It is actually by merging two disciplines, thermal physics and material sciences that one can predict the existence of these oceans.
3a/ Neptune and Uranus contain large amounts of carbon in their atmosphere.
Neptune and Uranus are believed to hold around 2% of their volume as methane (methane is CH4 so it contains 75% of carbon atoms from which are built up diamonds). Jupiter and Saturn hold around 0.3 to 0.5% of methane. These lower concentrations do not exclude the existence of diamond oceans near their core nor diamond rain. It just makes the phenomena a little less likely to occur.

3b/ What are diamonds?
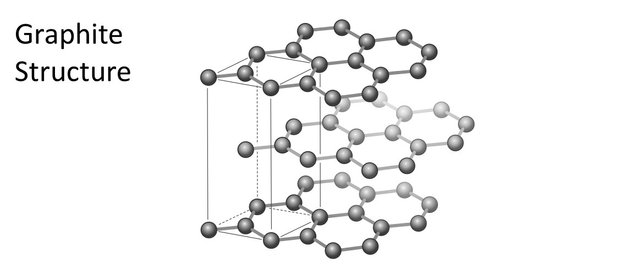
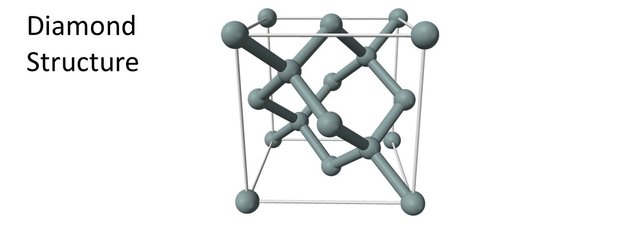
Carbon atoms can organize themselves in various ways. The two most common ones are the graphite structure and the diamond structure.
A carbon atom contains 4 valence electrons. That means that it can create a maximum of 4 bonds with its neighbor.
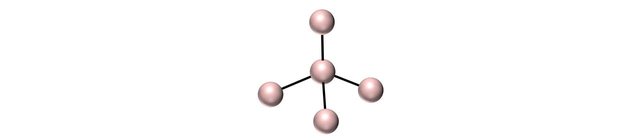
The graphite structure is the most stable one. It consist of 2-dimensional layers of carbon atoms that bond to each other via three bonds. This leaves one electron by itself that is therefore free to move around (graphite is conductor of electricity).
Diamonds consist of a 3-dimensional network of carbon atoms. All 4 valence electrons are used for bonding the atoms to each other. No electrons are free to move around. Diamond does not conduct electricity well (to be precise, it is actually a semi-conductor). For more details about how the the diamond structure lays out, please refer to episode 2.
3c The stability of diamond?
Under ambient conditions of temperature and pressure, graphite is the most stable form of crystalline carbon. Diamond is meta-stable.
What does this mean?
Consider a ball rolling down the slope of a hill. Why does it do that? It rolls down because it is attracted by the gravitational force. By decreasing its height, the ball decreases its potential energy. Nature loves doing stuff like that!
Suppose it arrives at position 1 and that it is not going fast enough to go over the crest at position 2. It oscillates a little around and then settles in position 1. One could say, the ball's position is stable. Not so fast…
I give it a nudge towards the right, it will start moving again. I give it a nudge energetic enough so that it can go over the crest at position 2. That makes it roll down position 3 where it finally settles for good.
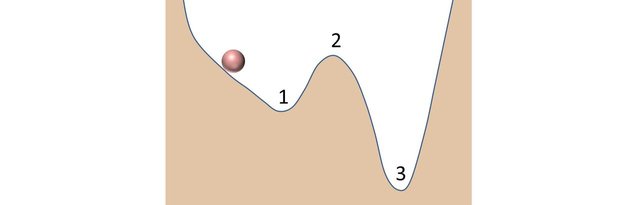
Position 3 is the lowest position in terms of potential energy: it is the most stable position (or state). Position 1 is not the most stable position as the ball can find a lowest energy position that can be reached if enough energy is given to it (such energy is called activation energy). Position 1 is said to be metastable. Note that position 2 can also be seen as metastable, but just a tiny bit: if the ball was at equilibrium at this point, any fluctuation would make the ball roll towards position 1 or 3.
The position of the ball represents the energy state of the crystalline structure. If the ball is moving, it means that the structure is still arranging itself, trying to find the lowest energy configuration it can. When it is in a stable or metastable state, atoms have stopped rearranging themselves and the structure is fixed.
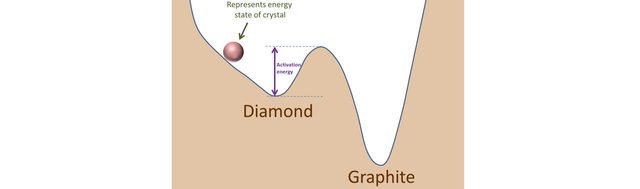
Graphite is stable, it corresponds to position 3. Diamond is metastable it corresponds to position 1.
Let’s take a diamond. It appears stable at ambient temperature and pressure. Let’s heat it. The energy state of the diamond will increase because we are providing heat to it (the ball rolls up the hill). If the amount of energy we bring to the crystal is higher than the activation energy (the ball passes the crest at position 2), then the diamond will turn into a more stable form: graphite (the ball rolls down towards position 3) .
In air, 700 C are sufficient to turn diamond into graphite. In a vacuum, the temperature must reach 1700C for the process to be significant.
So, it appears that melting diamond is an impossible task: Diamond decomposes into graphite before being able to reach a melting temperature. Besides, temperatures that were reached during our expedition inside Neptune were much higher than these, several thousands of degrees (Estimation is around 5000K)… So how come did we end up swimming in an ocean of liquid diamond ???
It is the huge pressure inside Neptune (about 7 million times the atmospheric pressure on Earth at ground level) that allows the magic to happen...

3d/ The role of pressure
The density of graphite is around 2.3 grams/cm3, that of diamond is 3.5 g/cm3. Hence, the diamond structure packs 50% more atoms in the same volume than graphite.
In a high pressure environment, atoms will try to organize themselves to take the less place possible: pressure will tend to force a carbon structure to take a diamond form. This means that under high pressure conditions, the structure of diamond is favored energetically compared to graphite. You can see it as if the 2 valleys were moving in height relatively to each other depending on the pressure conditions:
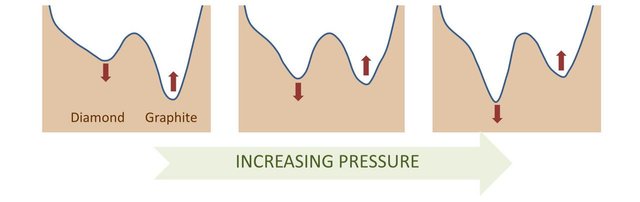
3e/ What are the conditions at which the diamond form is the most stable ?
As we have seen in the previous figure, increasing temperature gives energy to the ball that can then roll up the crest and pass from one dip to the other. Increasing pressure will make the diamond structure more and more stable because diamond is more compact than graphite. This behavior can be summarized in a graphic that is called a phase diagram.
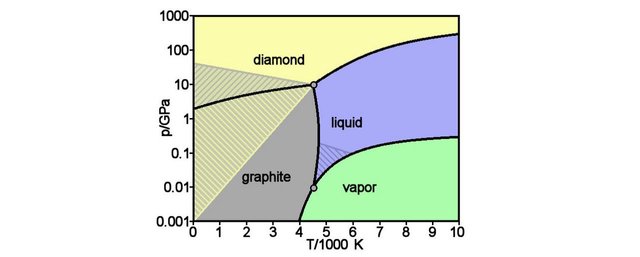
The theoretical phase diagram for carbon predicts within which ranges of pressures and temperatures a given carbon phase is stable. The hashed zones represent regions where there are two valleys and a crest: both graphite and diamond are either stable or metastable, and therefore can coexist. Non-hashed zones imply that only one phase is stable. For information, 1 GPa corresponds to a pressure of 10000 times the atmospheric pressure on Earth at ground level.
According to the phase diagram, Carbon at high temperature can only exist as graphite unless subjected to ultra-high pressures. In such conditions, the diamond structural configuration is the only stable form.
3f/ What about the liquid form of diamond?
As diamond becomes graphite at high temperature up to pressures around 10-100GPa, liquid diamond could never be observed melting… up to recently. Scientists finally have been able to reproduce in the laboratory pressures of the order of 1000GPa while simultaneously applying temperatures of several tens of thousands of degrees.
The technique used is based on shockwaves. The sample is placed behind a material. Lasers are used to send ultra-short burst of energy with huge power to the material in front of the sample. The momentum of the material hit by the laser transmits to the sample under the form of a shockwave that consequently applies a huge pressure to the sample and heats it up to 100000K.

While the sample cools, the decrease of the sample's temperature is measured using black body radiation emitted by the sample. When you heat a body, it emits light. The hotter it becomes, the higher the frequency of the light: with increasing temperature, the body first becomes red, then orange, then yellow, green, blue etc… This is called black body radiation and allows determining a body’s temperature.
A plateau was observed in the decrease of temperature with time, corresponding to a heat of solidification. Because graphite cannot exist in these conditions, it can only be liquid diamond “freezing”. The pressure and temperature conditions obtained during these experiments are those found inside giant gas planets like Neptune or Uranus.
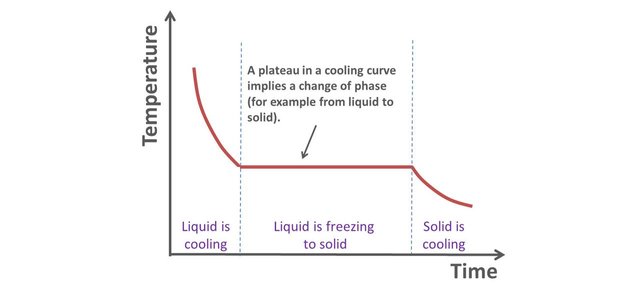
This research [source - pdf download] makes the existence of liquid diamond oceans surrounding the surface of their rocky core very plausible! In addition, scientist were able to measure other properties of the liquid phase. It appears that unlike most materials, solid diamond has a lower density than its liquid form. This is also the case for water. One can deduce that diamond icebergs floating on diamond oceans would be plausible too!

4/ Conclusion
Wow! What amazing science our scientists are able to develop nowadays! Scientific research is definitely not a dull activity. And when you think about the conclusions that such work imply... There is so much to be dazzled about.
Liquid oceans of diamonds surrounding the core of giant planets, with diamond flakes falling from the liquid water skies… What I wouldn’t give to go for a little water-current-surfing on such a sea, zigzagging between the diamond icebergs!

Previous episodes:
- Diamonds in The Sky - Part 1:
The story of how our sun will become a massive diamond floating in the sky. - Diamonds in The Sky - Part 2:
Clathrate Stars - Diamonds in The Sky - Part 3:
Diamond Oceans - Episode 1
Image Credits:
- The post's thumbnail was created by using pictures found on Pixabay: Ocean (link), Space background (link).
- The diamond structure is public domain and was created by Ben Mills (link)
- The graphite strcture was created by Jozef Sivek on wikimedia commons (link)
- Elements of the planets-diamond frieze were found on Pixabay and on wikimedia commons (link1, link2)
- The theoretical phase diagram for carbon was found on wikimedia commons (link)
- The shockwave laser beam picture was found on Pixabay (link)
- The picture of the diamond ocean with the iceberg was found on wikimedia common and authored "Visit Greenland from Nuuk" (link)
- All other illustrations were created from scratch by @muphy
Reference and text sources:
- Melting temperature of diamond at ultrahigh pressure. Eggert et al. (Nature Physics, Vol. 6, No. 1, 01.2010, p. 40-43.) (pdf download)
- https://en.wikipedia.org/wiki/Diamond
- https://en.wikipedia.org/wiki/RankineHugoniot_conditions
- https://en.wikipedia.org/wiki/Extraterrestrial_diamonds#Solar_System
- "Outer planets may have oceans of diamond" by Eric Bland (link)
- "It rains solid diamonds on Uranus and Neptune" by Sarah Kaplan (link)

Hi,
I’m @muphy (see intro post),
My life revolves around music production, teaching sciences, and discovery through travel.
You enjoyed that post? Resteem and Upvote!
You are interested in these topics? Follow me!
Really great post, thanks!
I love how you've broken it down to bite-size pieces otherwise I fear I'd be left behind!
How long till some corporation flies out to mine these :p
Thank you for your kind words @tfcoates, and I am glad you stayed onboard!
You know it may be a while! It would probably be cheaper to go mine diamonds on a carbon planet orbiting another star than to visit the depth of Neptune. No known material would resist such temperature and pressures!
This is, in my opinion, absolutely awesome.
Cheers, I am glad you enjoyed the voyage :-)
Beautiful theme worth reading
nice
Congratulations @muphy, this post is the tenth most rewarded post (based on pending payouts) in the last 12 hours written by a Superuser account holder (accounts that hold between 1 and 10 Mega Vests). The total number of posts by Superuser account holders during this period was 1454 and the total pending payments to posts in this category was $12634.59. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Nice post :)
I'm proud of you, keep on working.
Not my intention to beg, but if you please please upvote my post as well. Nothing wrong if we support each other. I know if you are a good person and help each other: )
my support let me add the spirit :)
I wait your presence in my post :) @muphy
https://steemit.com/aceh/@mahyul/jalan-jalan-ke-waduk-jelekat-lhokseumawe-8edd295ed6fc1