How is The History of Chloroform (chemistry)?
His background as a lower class led to his ideal as a doctor foundered. Not satisfied with the fact, he went in and out of the hospital and studied drugs illegally.
With his medical knowledge, he decided to migrate to America. Even though he did not study medicine professionally, the immigration agency gave him a job as a nurse in a nursing home.
Within a period of 4 months, there were 17 patients who died in the nursing home. This large amount caused suspicion. After police officers conducted an investigation, with a strange appearance, Frederick Mors came and claimed to have killed 8 nursing home patients.
The history of medicine shows how societies have changed in their approach to illness and disease from ancient times to the present. Early medical traditions include those of Babylon, China, Egypt and India. The Indians introduced the concepts of medical diagnosis, prognosis, and advanced medical ethics. The Hippocratic Oath was written in ancient Greece in the 5th century BCE, and is a direct inspiration for oaths of office that physicians swear upon entry into the profession today. In the Middle Ages, surgical practices inherited from the ancient masters were improved and then systematized in Rogerius's The Practice of Surgery. Universities began systematic training of physicians around 1220 C.E. in Italy. During the Renaissance, understanding of anatomy improved, and the microscope was invented. Prior to the 19th century, humorism (also known as humoralism) was thought to explain the cause of disease but it was gradually replaced by the germ theory of disease, leading to effective treatments and even cures for many infectious diseases. Military doctors advanced the methods of trauma treatment and surgery. Public health measures were developed especially in the 19th century as the rapid growth of cities required systematic sanitary measures. Advanced research centers opened in the early 20th century, often connected with major hospitals. The mid-20th century was characterized by new biological treatments, such as antibiotics. These advancements, along with developments in chemistry, genetics, and radiography led to modern medicine. Medicine was heavily professionalized in the 20th century, and new careers opened to women as nurses (from the 1870s) and as physicians (especially after 1970). Wikipedia source
One of the most puzzling things about this case is how relaxed and polite Mors answered all the questions asked by the judge. Initially, he used arsenic to kill his patients.
However, this method causes the patient to feel tortured, in pain, produce terrible symptoms and can last for a few days before death. Mors said that the way was to torture the victims and themselves as perpetrators.
With the pharmaceutical knowledge he obtained from a hospital in Austria, he found that chloroform with a sweet smell (such as saccharin) and also volatile (volatile) was able to anesthetize without leaving suffering to its victims. In his confession, it was said, "Everything (the murder with the help of chloroform) occurs scientifically and without pain."
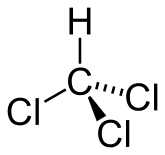
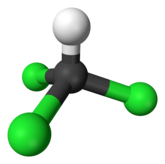
Chloroform, or trichloromethane, is an organic compound with formula CHCl3. It is a colorless, sweet-smelling, dense liquid that is produced on a large scale as a precursor to PTFE. It is also a precursor to various refrigerants. It is one of the four chloromethanes and a trihalomethane. It is a powerful anesthetic, euphoriant, anxiolytic and sedative when inhaled or ingested. Wikipedia source
This story is like a dark history of chloroform chemical compounds. However, chloroform was actually known as a magic compound that helped save thousands of people on the operating table. Let us know more about this compound so that it is not misused.
Chloroform in the medical world
His name was James Young Simpson, a doctor in Edinburgh who conducted research on drugs that were better for use at the operating table and during childbirth. In an experiment with his co-workers, he had an accident in the laboratory that caused a spill of chloroform.
After inhaling chloroform vapor in the laboratory room, in just two minutes they had lost consciousness. Thirty minutes later, when they realized, they understood that they had found a chemical solution to replace ether for anesthesia.
In 1847, Scottish obstetrician James Y. Simpson was the first to demonstrate the anaesthetic properties of chloroform on humans and helped to popularise the drug for use in medicine. By the 1850s, chloroform was being produced on a commercial basis by using the Liebig procedure, which retained its importance until the 1960s. Today, chloroform — along with dichloromethane — is prepared exclusively and on a massive scale by the chlorination of methane and chloromethane. Wikipedia source
Chloroform is a colorless, volatile, liquid derivative of trichloromethane with an ether-like odor. Formerly used as an inhaled anesthetic during surgery, the primary use of chloroform today is in industry, where it is used as a solvent and in the production of the refrigerant freon. Acute chloroform toxicity results in impaired liver function, cardiac arrhythmia, nausea and central nervous system dysfunction. As a byproduct of water chlorination, chloroform may be present in small amounts in chlorinated water (NCI04). Chloroform is found in spearmint. Indirect food additive arising from adhesives and polymers Chloroform is a common solvent in the laboratory because it is relatively unreactive, miscible with most organic liquids, and conveniently volatile. Chloroform is used as a solvent in the pharmaceutical industry and for producing dyes and pesticides. Chloroform is an effective solvent for alkaloids in their base form and thus plant material is commonly extracted with chloroform for pharmaceutical processing. Pubchem source
This chemical is widely known for the next five decades. However, chloroform has an anesthetic mechanism that was not well understood at the time. In some operations, there is death caused by chloroform. In 1932, at least the ratio of deaths from the use of chloroform in anesthesia to the number of operations was 1: 3000.
This figure is much higher than the previous anesthesia material, either, which is only 1 case in 14,000 uses. The popularity of chloroform in the medical world eventually declined and disappeared after the discovery of nitrogen oxide and hexobarbital gas as an anesthetic.
The Identification of chloroform
The chemical formula of chloroform is CHCl3, which belongs to the class of haloalkane compounds. The scientific name is trichloro-methane. Because the compounds are composed of alkane chains and halogen groups, we will find it difficult to recognize them when they enter the body of living things.
Chloroform containing deuterium (heavy hydrogen), CDCl3, is a common solvent used in NMR spectroscopy. It can be used to bond pieces of acrylic glass (also known under the trade names Perspex and Plexiglas). Chloroform is a solvent of phenol:chloroform: isoamyl alcohol is used to dissolve non-nucleic acid biomolecules in DNA and RNA extractions. Chloroform is the organic compound with formula CHCl3. Chloroform source
This is one reason why killing using chloroform was very popular in the past. Mors, who had killed 7 victims using chloroform, could not be jailed because of the lack of results of a police autopsy that could prove the use of chloroform in the bodies of the elderly people he had killed.
The value of medical expertise is apparent in the screening process. In one county, for example, 8,000 cases are reported to the medical examiner's office, but only 2,000 are accepted. Screening, which eliminates three-fourths of potential cases, must be handled in a scientifically defensible manner by people with medical training, knowledge, and objectivity. The medical examiner's office is especially important in subtler cases of criminal activity, where there is a possibility of a missed homicide. Such cases often are not aggressively pursued by either police or non-medical coroners. Confronted with the death of a 30-year-old woman, who dies apparently of a heart attack, a lay coroner would most likely not do an autopsy, but a medical examiner would, given its medical implausibility. Similarly, many lay coroners do not autopsy burned bodies, but a medical examiner would investigate the possibility of homicide masked as an accident. By interviewing, the medical examiner might uncover evidence of a crime. NCBI source
Until now, this compound still has potential danger because of its ease of use in cases of murder, confinement, and kidnapping. The Anesthetic effect that can occur in a short time and is very calm makes it very effective.
Another fact is that it's easy to get or produce this compound. Therefore, chloroform was quite popular in muggings and robberies in Indonesia in the 1990s.
Fortunately, autopsies for chloroform use cases have been very easy in this modern era. The trick is to crush the indicated body tissue containing chloroform, then distillate the steam, mix the resulting syrup with NaOH and benzene, then boil it.
If a yellow-greenish solution is formed, there must be a chloroform that plays a role there. Because it's so easy to identify chloroform at this time, don't try to use it to break the law!
Use of chloroform in the industry
Although chloroform is not known in the medical world, its use is still very much in the industry. Its effective nature as an organic solvent is one reason for its use in the industrial world. The pesticide industry still uses chloroform as the active ingredient in its products, but this usage is gradually reduced.
Based on research in recent years, there are indications that chloroform is a carcinogenic compound. Chloroform is currently used in the manufacture of Kloro-difluoromethane, CHClF2, cooling compounds in electronic products, air conditioners, and refrigerators. Another use is in the laboratory in extracting DNA and RNA.
Then, what about the fate of Frederick Mors in the case that was told at the beginning? Because the failure of the police to prove the content of chloroform in the victim's body, Mors only received a sentence of exile in a mental hospital. In this verdict, the judge reasoned that Mors' mental health was disrupted.
His recognition of killing with chloroform could not be proven at autopsy so he was not sentenced to prisoner/death. However, sometime later, Mors escaped from a mental hospital and his fate was unknown. That is why forensic chemistry is an important branch of science to uphold legal justice.
Reference:
History of Medicine Wikipedia source


Thanks for using eSteem!
Your post has been voted as a part of eSteem encouragement program. Keep up the good work! Install Android, iOS Mobile app or Windows, Mac, Linux Surfer app, if you haven't already!
Learn more: https://esteem.app
Join our discord: https://discord.gg/8eHupPq