Air-Fuel Ratio: Most Important In the Combustion of Hydrocarbon Fuels with Sample Problem Solving
Hi Everyone!
Today, I will share my knowledge regarding one of the most important aspects in the field of Combustion which is about air and fuel and to be specific the air-fuel ratio. Additionally, I will share my shortcuts in calculation which I always do whenever I met problems relating to air-fuel ratio wherein it uses alkanes as its fuel.
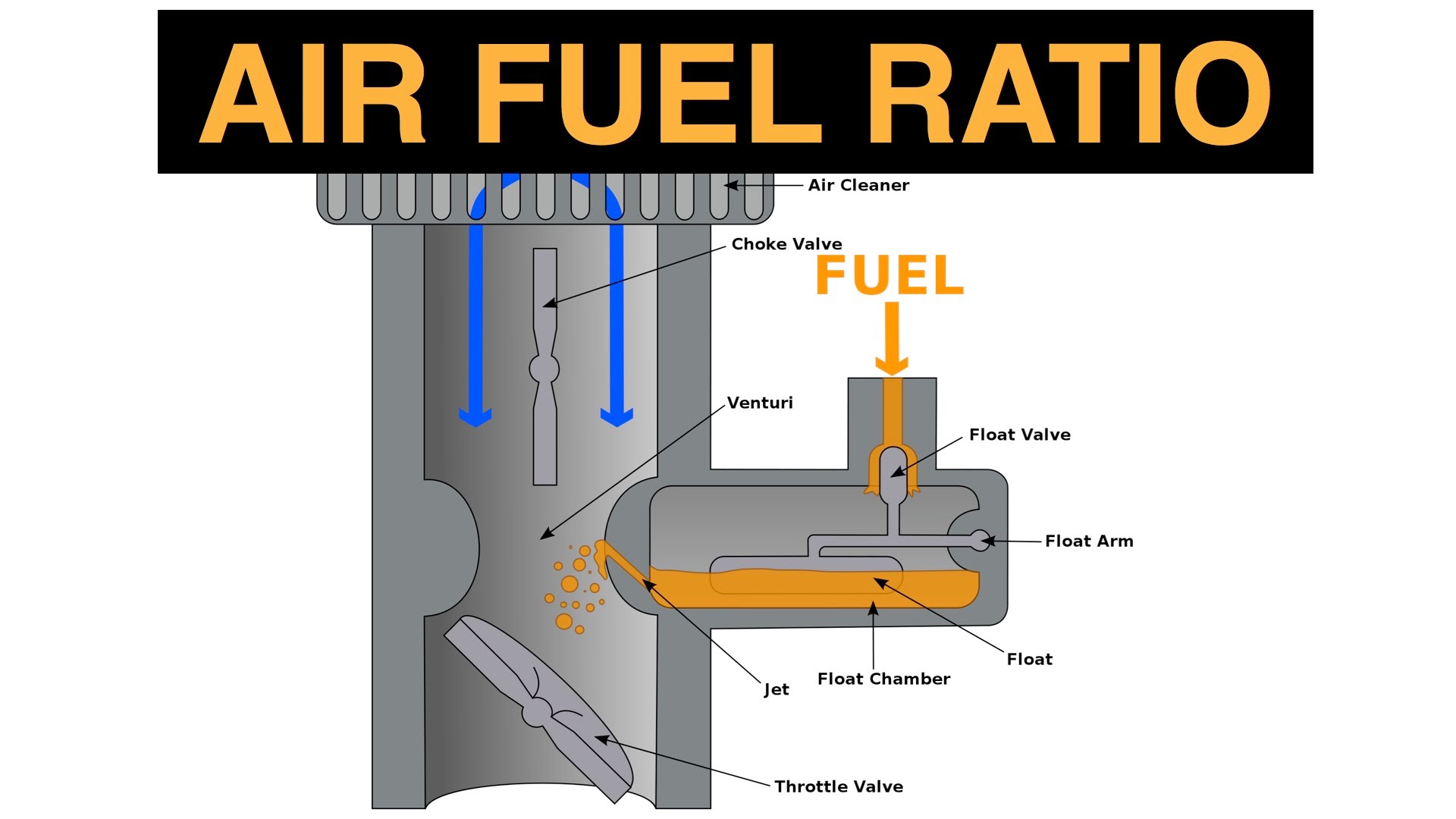
The Importance of Air-Fuel Ratio
Ever noticed the different colors of smoke that is being exhausted by the different automobiles along the streets? We often saw “black” smokes being exhausted by automobiles or commercial trucks. We also see that some automobiles are exhausting “white” smokes and sometimes we see high-end cars wherein we almost see little to none exhaustion of flue gases and exhaust gases. And sometimes we see a colorization of “blue” to the flame which we often see in our gas stoves.
Those aforementioned colors of smoke being exhausted by different automobiles have indications which are as follows:
- Black
- This is an indication that there is an incomplete combustion of both air and fuel which is a result of insufficient amount of air being supplied to the engine or burner. The black smoke is an indication also of the emission of Carbon monoxide (CO) which is a very harmful gas.
- White
- This is an indication of complete combustion of both the air and fuel which is a result of the excess amount of air being supplied to the engine or burner. The white smoke is the by-product of the combustion wherein it is express in terms of excess Oxygen gas, O2.
- Blue
- This is an indication of a complete combustion of air and fuel.
- This is an indication of a complete combustion of air and fuel.
And based on these colors, we can definitely say that for example, there is a problem with the engine or burner that is used to burn both the fuel and the air needs to be cleaned or worst, be replaced with a new one.
How do we obtain the Air-Fuel ratio needed to burned a mole of fuel?
For this blog post, since I am will be using alkanes as the fuel being burned by the engine or burner, I will be discussing the method in obtaining the theoretical and actual air-fuel ratio needed to burned a mole of fuel.
So let us start with the Theoretical Air – Fuel Ratio for hydrocarbon fuels such as alkanes. And since hydrocarbons are compounds composed of Carbon (C) and Hydrogen (H) atoms, expect that there are Carbon dioxide (CO2) and Water (H2O) as products of combustions and additionally, Nitrogen gas (N2) since air is expressed majorly as a mixture of oxygen and nitrogen gases.
So here is the general equation needed in order to obtain the Air – Fuel ratio:
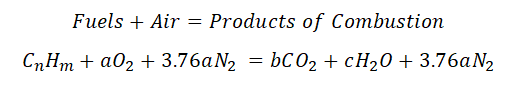
wherein:
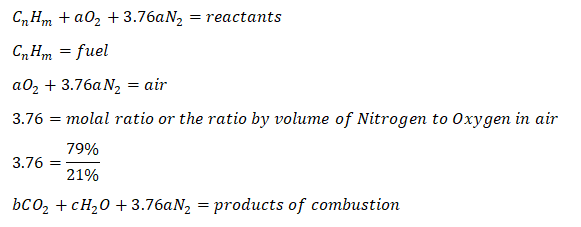
Furthermore,

So those are the important parameters needed in balancing the chemical reaction between the air and the fuel, by now, we will proceed to the general formula in obtaining the air-fuel ratio:
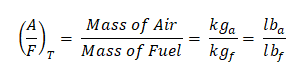
where:
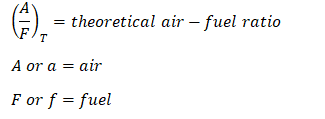
Furthermore,
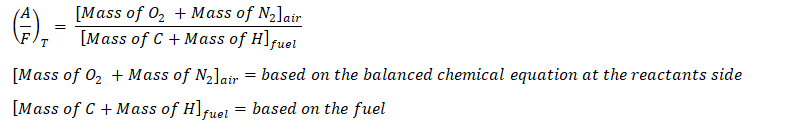
Note: This formula can also be used for the theoretical air-fuel ratio, since the idea of mass of air divided by the mass of fuel is generic.
Then,
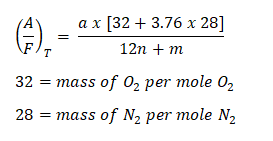
And as for the actual air-fuel ratio wherein there is an excess of air,
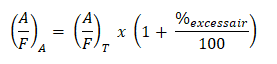
where:
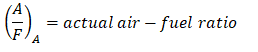
Additionally, we have a new balanced chemical equation wherein it takes into account the excess air.
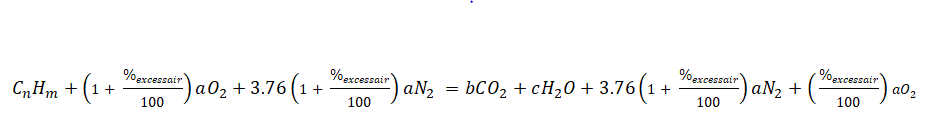
So those are the equations and formulas needed to obtain the air-fuel ratio for hydrocarbon fuels whether it is with excess air or not.
Sample Problem Solving
Well, let us try solving a sample problem wherein theoretical air-fuel ratio and actual air-fuel ratio is both used and additionally, I will show my shortcut for it (applicable only for alkanes), which is very useful if you are going into quizbowl or in an examination or board examination.
So here is the sample problem;
A fuel oil is burned with 50% excess air. The fuel oil used is dodecane wherein it has chemical formula of C12H26. Solve for the actual air-fuel ratio.
Note: I made this sample problem.
Solution:
The first thing to do is to balance the chemical equation by using the chemical equation wherein excess air is taken into account.
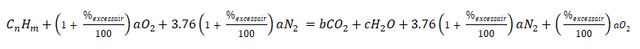
Second, substituting the values for n and m,
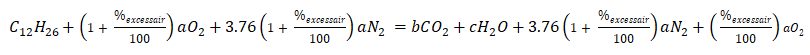
Next is obtaining the values for b, c, and a.
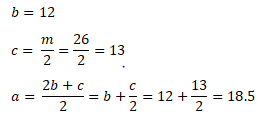
Substituting the values we have obtained,
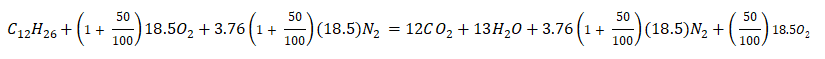
Then, we now have a balanced chemical equation,
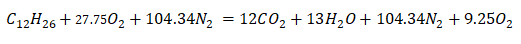
If you've noticed that 9.25 moles of O2, it is actually the 50% excess air, or mathematically the 50% of 18.5 moles of O2 needed to burn 1 mole of C12H26.
By now, we can proceed in obtaining the actual air-fuel ratio; by using the formula as seen below,
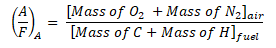
Take note that this formula can also be used for both the actual and theoretical air-fuel ratio since the mass are derived from the balanced chemical equation of both the reactants and the products.
And substituting the values to the formula,
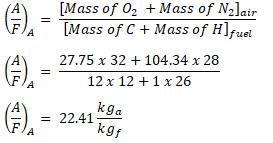
And we obtained an air-fuel ratio of 22.41 kga or lba per kgf or lbf.
So as mentioned in the early part of this blog post, I will share my shortcut formula whenever I meet problems that require to solve the air-fuel ratio needed to burn a mole of fuel to be specific those alkanes. So let me start introducing my shortcut formula,
For Alkanes
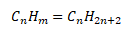
So,
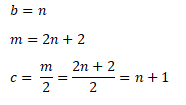
Therefore,
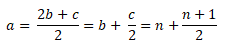
Then, substituting the derived value of a in terms of n, in the equation in calculating the air-fuel ratio,
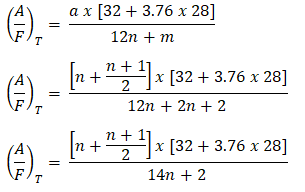
As you can see, we now have a formula for the air-fuel ratio wherein the only parameter it takes into account is the number of Carbon (C) atoms in a hydrocarbon to be specific those alkanes, by which this shortcut formula is only applicable.
And here is the shortcut formula, wherein it incorporates and takes into account the excess air which is expressed in percent.
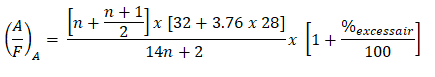
Finally, substituting the value for n and the excess air which is expressed in percent. And for dodecanes, number of Carbon atoms is 12, which is the n.
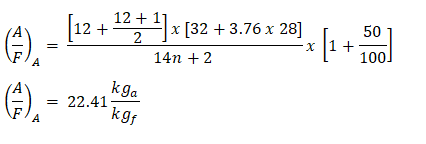
And as you can see we arrived with the same results, wherein the actual air-fuel ratio is 22.41 kga or lba per kgf or lbf.
I was able to derived that formula when I was a 4th year mechanical engineering student and I am very grateful for my high school alma mater since I become a part of their DOST-ESEP (Department of Science and Technology - Engineering and Science Educational Program) wherein we were offered with Basic and Advance subjects for both Chemistry and Physics. I learned alkanes in my Advance Chemistry subject and I have to be honest, Chemistry happened to be my favorite subject especially when we were discussed about hydrocarbons and other aromatic hydrocarbons wherein each one of us in my class that time is assigned to a specific hydrocarbon and needs to discussed it in front of the class and everyone need to know the naming and the chemical structure of the organic compound. And moving back to my Combustion Engineering subject, my instructor accepted my shortcut since I explained it well to my classmates about alkanes, their naming and their nomenclature.
I guess that would be all for this blog post of mine which is about the air-fuel ratio needed in burning a mole of fuel and the effects of air-fuel ratio after combustion takes place.
Thank you for spending your time reading blog post.
Much love and respect.
Ace | @josephace135
Your blog has received an upvote from the communal account of Steemph.antipolo for being an active discord member and as an active community member. Keep up the good work and best of regards. Keep on Steeming!
You can get a support by joining our discord channel and gain votes from
our curators. Join our discord now
https://discord.gg/7w3hJqw
If you would like to support steemph.antipolo project you can help by degegating your spare SP to us, just click the link below.
50 SP 100 SP 200 SP 300 SP 400 SP 500 SP 1000 SP
You have been upvoted by the @sndbox-alpha! Our curation team is currently formed by @jeffbernst, @bitrocker2020, @jrswab & @teachblogger . We are seeking posts of the highest quality and we deem your endeavour as one of them. If you want to get to know more, feel free to check our blog.
Congratulations! This post has been upvoted by the communal account, @steemph.cebu by josephace135 being run at Teenvestors Cebu (Road to Financial Freedom Channel). This service is exclusive to Steemians following the Steemph.cebu trail at Steemauto. Thank you for following Steemph.cebu curation trail!
Don't forget to join Steem PH Discord Server, our Discord Server for Philippines.
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josephace135 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.