Exploring Polyamides: Kevlar and Nylon
Copolymers Kevlar and nylon both use amide bonds to link monomers together; each plays very distinct roles in the first and second "polymer centuries"
Less than a century ago, a lightweight material strong enough to stop a bullet would have fallen firmly into the realm of science fiction; and attempts at bypassing the silkworm to create manmade sheer-and-sexy stockings were depressingly mired in baggy cellulose-based fabrics. It took the trusty amide bond and some creative organic chemistry to solve the former problem with Kevlar and the latter with nylon.
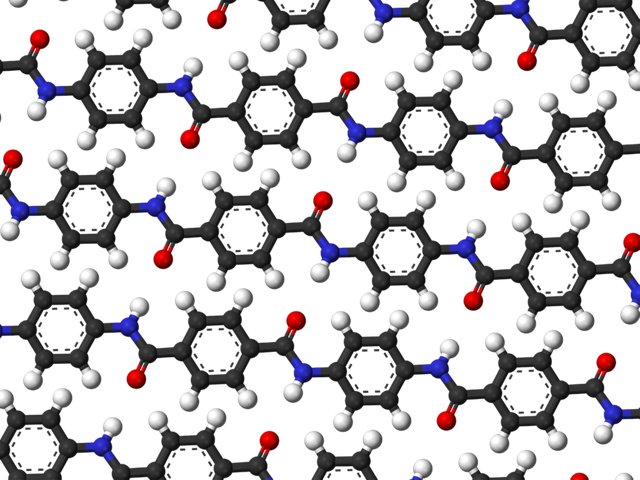
The Birth and Development of Nylon: The Definitive Artificial Silk
The first artificial silk was made in the late 1870s by Count Hilaire de Chardonnet, who noticed he could pull long threads from a spilled photographic solution known as collodion. Unfortunately, the polymer in collodion was actually nitrocellulose, so Chardonnet silk had a distressing tendency to burst into flame or explode, giving a whole new meaning to the phrase “a dress to die for.”
One hundred and twenty years later, London experimenters Charles Cross, Clayton Beadle, and Edward Bevan synthesized viscose silk by forcing high-viscosity liquid through a spinnaret. The cellulose polymer produced in this fashion absorbed moisture, though; and saggy fake-silk stockings hardly qualified anybody for the best-dressed list.
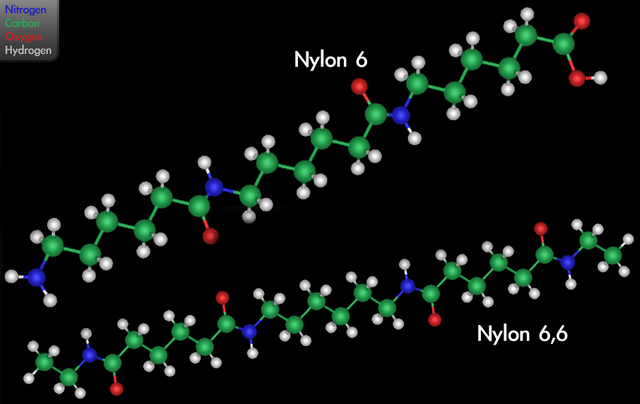
Finally, in 1938, Harvard University organic chemist Wallace Carothers polymerized nylon for Du Pont. Using apidic acid (hexanedioic acid) and 1,6-diaminohexane, he made “nylon 6,6” with a simple condensation reaction whose product brilliantly mimicked many of the properties of silk.
Like nylon, natural silk possesses amide bonds, but is a true pleated-sheet protein with chains mostly composed of the amino acids serine, alanine, and glycine. While nylon perhaps did not have exactly the same sheen as silk, it nonetheless turned out to be almost perfect for stockings since it fitted tightly to the leg, was relatively durable, and could be made available to the public at an affordable price. Within one year of nylon’s birth, 64 million pairs of hosiery made from it hopped off the shelves.
There are now two other types of nylon fibers: nylon 6, produced by the self-condensation of 6-aminohexanoic acid (the original monomer is caprolactam; its ring opens when it polymerizes); and nylon 6, 10, polymerized from 1,6-diaminohexane and decanedioic acid. Modern industrial processes generally use high heat and pressure to efficiently produce the polyamide. In the modern process there is substitution of more reactive dichlorides (such as hexanedioyl dichloride) for dicarboxylic acids. This substitution causes hydrochloric acid rather than water to be released when the amide bond forms.
The Many Uses of Nylon
Du Pont contributed greatly to the American World War II effort. Nylon proved useful for many military applications such as weather balloons, parachutes, ropes, tire cords, and mosquito netting. In contrast, Nazi Germany invested heavily in cellulose-based rayon fabrics.Rayon doesn’t provide much protection in the bitter cold and the uniforms made from this material may have contributed to Germany's failures on the Russian front during harsh winter weather.
Nylon is still used to make much more than just the pantyhose ubiquitously referred to as “nylons.” Various types of clothing, the inner structure of automobile tires, boat sails, upholstery, and carpets all owe a profound debt to Du Pont and Carothers’s very practical and useful polyamide. In the world of beauty, nylon yields good synthetic resins, thickens cosmetics and makes them opaque, lengthens eyelashes, and soaks up superfluous facial oil. During the 1950s, it even became the first so-called engineering plastic and was convenient for constructing machine cogs and bearings.
Of course, despite its wartime successes, it can’t stop a bullet. But then, what on earth ever could? Oh, yeah…
Kevlar: The Super-strong Aromatic Polyamide
Also made by Du Pont, Kevlar is the registered trademark of a specific type of aromatic polyamide copolymer often called an aramid. Composed of para-phenylenediamine and terephthalic acid (or its corresponding acid chloride), Kevlar was discovered in 1965 by Stephanie Kwolek, but various synthesis problems delayed its debut. It finally and fittingly hit the market in 1982, exactly fifty years after the creation of the last bullet-impervious material (i.e., Superman’s chest - of course, just a jest! On a more serious note, the bullet-deflecting material polymethyl methacrylate was first synthesized in 1931 by German chemist Dr. Otto Rohm, but did not reach the general public until some years later)
The unusual benzene-ring-containing backbone of Kevlar and the hydrogen bonding between the nitrogen-containing group and the oxygen of the carbonyl groups on adjacent chains contribute to its tremendous structural regularity. Kevlar’s strands are powerfully attracted to one another, forming tightly-packed sheets rather than the ordinary mangled mess familiar to polymer enthusiasts everywhere.
The main drawback to Kevlar is its rather exorbitant price. Kevlar also presents other dilemmas since it is difficult to paint, cut, or drill; exhibits poor compression strength; displays susceptibility to degradation/decomposition with prolonged exposure to ultraviolet light. Despite these negative reasons, Kevlar is well worth its cost.
Kevlar is five times as strong as steel, exceedingly lightweight; does not twist or tear and garments made from it are lightweight and do not burden the wearer. Kevlar is usually not affected by solvents, shows no signs of weakness after immersion in boiling water or liquid hydrocarbons even after years of exposure. Kevlar will dissolve in pure sulfuric acid, but so do many materials. Kevlar does not become brittle at very low temperatures, resists flames and weathering, and is nicely flexible for a variety of uses. Kevlar is most important for its effectiveness as body armor and bombproof material and this is due to the super-strong polymer mesh’s absorption of energy from bullets and blasts.
Currently, a lighter, thinner, more flexible version of body armor is being developed: 10 layers of Kevlar are combined with a Shear Thickening Liquid, then placed over 31 layers of untreated Kevlar. The severe bruising and internal trauma resulting from gunshot impact will be reduced by this improvement; physical fatigue caused by heavy armor should also be cut considerably.
The Many Uses of Kevlar
Kevlar’s tensile strength makes it a superior and effective defensive device for the walls of panic rooms and aircraft engine compartments, vests, flak jackets, head gear and related equipment for soldiers and police officers. Kevlar is used for mining conveyor belts, fire-resistant clothing, safety gloves, chemical hoses, tear-proof sails for boats, storm-resistant buildings, mooring ropes for offshore oil rigs, tankers, and even the Mars Pathfinder landing apparatus.
Kevlar is not limited to the above herculean tasks alone. Kevlar assists people to enjoy the sporting life in: laminates that coat racecar drivers’ survival compartments, tennis and badminton racquets, skis, running shoes, puncture-resistant kayaks and canoes. Forty years ago, Kevlar was widely used in Formula 1 racing tires, but the aforementioned problems have led to it being phased out of this application to a certain extent. Kevlar has its artistic side, too, when employed in musical instrument components such as reeds and drum heads
Nylon and Kevlar truly exhibit the fun “Merlin the Magician” quality of polymer chemistry. Both use not-so-amazing monomers and the same taken-for-granted amide bond found in all peptides or proteins to produce macromolecules that beat nature at her own game. Humankind's chemical advances have altered society’s perceptions and expectations. Nylon and Kevlar as "un-natural" results of laboratory toil and talent save lives, increase human pleasure/safety in games, make people look better, and quietly change the world one molecule at a time.
Sources
- Barrie, Alison, "Forget Kevlar! Liquid body armor hardens on impact," FOX.
- Caswell, Adam, "Other Uses of Kevlar," ffden-2.phys.uaf.edu.
- Clark, Jim, "Polyamides," chemguide.co.uk.
- Daintith, John, editor, A Dictionary of Chemistry, Third ed., Oxford University Press.
- Emsley, John, Molecules at an Exhibition, Oxford University Press.
- Le Couteur, Penny and Burreson, Jay, Napoleon’s Buttons: 17 Molecules That Changed History, New York: Jeremy P. Tarcher/Penguin.
- "Macrolab Experiment Making Nylon 6 and 6, 10," USM Polymer Science Online Laboratory.
- Winter, Ruth, A Consumer’s Dictionary of Cosmetic Ingredients, New York: Three Rivers Press.
@resteemator is a new bot casting votes for its followers. Follow @resteemator and vote this comment to increase your chance to be voted in the future!