Spectroscopy Series VOL. 11: AAS Atomic Absorption Spectroscopy
Welcome again to another installment of my spectroscopy series. This time I will talk a little about the technique of atomic absorption and one of its most used characterization methods with is the absorption in flame.
But before starting the content of this publication I suggest you visit my previous deliveries:
Vol.1 Vol.2 Vol.3 Vol.4 Vol.5 Vol.6 Vol.7 Vol.8 Vol.9 Vol.10This technique of spectroscopy is undoubtedly one of the most powerful that has been created throughout history, its creation dates from the middle of the last century by the 50s. This technique covers three types of techniques that are based on absorption, emission and fluorescence, where a certain atomic vapor produces a radiation that is reflected in a spectrum of a specific material.
I have never had the opportunity to work with this technique, but as a lover and to know about the subject I have enough knowledge about it despite the little experimental experience. Although some other techniques such as infrared spectroscopy are also based on absorption. To continuation I would like to share a theoretical compilation where I can make known what atomic spectroscopy is about. First of all, I'm going to delve into the atomic absorption technique.
The atomic absorption spectroscopy known in its acronym as (AAS) is a technique widely used by chemists, used to determine concentrations of many materials. The great thing about this technique is that it can characterize approximately 70 different elements in a solid sample or solution, ideal for applications in drugs, toxicology and biophysics.
This type of spectroscopy (AAS) is divided into three methods which are: Graphite Furnace, Hydride Generation and the best known Llama. I could say that these methods are the three different types with which a material can be atomized.
Physical principle
Basically it consists of absorbing a certain wavelength, the radiation is absorbed by atoms that are located in the energetic levels, where the photons generate a difference of energy corresponds to the value of these. In a previous post I spoke about Beer-Lambert's law, where absorbed photons are determined by this law.
In this publication we will focus on explaining the atomization in flames where a source that emits radiation with a wavelength equal to that of the analyte is used. Subsequently, one of the selected lines must cross the flame where the analyte atoms whose main function is to absorb the emitted radiation are concentrated.
We can say that the electrons that are inside the atomizer are directed to higher energetic levels for a determined time through the absorption of the analyte that serves as a source of radiation, that is to say, an energetic quantity determined by the wavelength. All this emitted energy is the transition generated by the electrons to be transferred from one orbital to another, where each wavelength belongs to an element or material analyzed.
If we know the amount of energy that can be generated by the flame and the rest that is in the detector of the spectrometer, from all this it is possible to calculate the transitions that are generated by means of the Beer-Lambert equation for the purpose to obtain the chemical concentration of the characterized element or material.
Flame absorption spectroscopy
In order to carry out this type of measurements with the AAS in Llama we must know first what are the components that constitute a spectrometer, which are the following:
First, we must have an emission source that is responsible for transmitting a line that makes the atomic transition from one orbital to another of the material that we want to characterize.
A detector that is responsible for capturing all the signal emitted by the source and this has the ability to proportionally transform the electromagnetic signals to electrical, ie the radiation intensities emitted from the material.
We must also have a burner that allows the formation of atoms from the components of the analyzed material. It is important to mention that the temperature reached by the material is given by the effect of the combustion and reaction of the sample.
The device incorporates a Nebulizer, which is used to atomize and remove solvent material from the sample to be analyzed, which subsequently tends to form tiny drops so that it can exert a very efficient atomization by aspiration of the sample in a specific liquid solution.
An amplifier, whose main function where is to amplify the signal emitted by means of the detector of the intensities of the radiation, then this signal will be processed by means of an electronic circuit. It should be noted that the amplifier plays an essential role, since if we have an inefficient component, the spectra can not be obtained in a visible way.
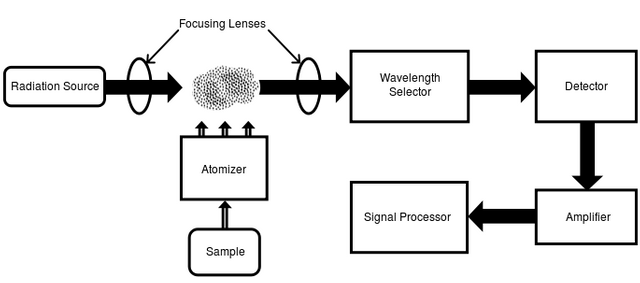 Diagram showing the components of the flame spectrometer[Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0)]
Diagram showing the components of the flame spectrometer[Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0)]Optical system composed of a monochromator, where it can perform the work of separating the radiations emitted in the system from the wavelengths for better performance and sampling quality.
And the most important component of the EAA is the atomizer, there are different types of atomizers, the most used are the turbulent flow lighters and the laminar or premix flow lighters. Next, I will explain the atomization process in the AAS.
At the end of the analysis of the material, we need a system where we can read the current signal, this signal will be converted into a spectrum that is the fingerprint of the material and what we are looking for from the beginning. This reading system is coupled to the spectrophotometer that finally carries the signal to the computer that through specialized software we visualize the spectrum obtained.
Flame atomization process
Everything begins with a flow of gas that is transported from the sample of the material that you want to analyze to a hot region, where it gives rise to the atomization process, this process is developed by several stages. The first corresponds to the desolvation, in which the solvent evaporates and then produces a solid molecular aerosol finely in salt particles. The dissociation of the molecules leads to the formation of an atomic gas, in turn the atoms can dissociate into ions and electrons. It should be mentioned that the speed of this process depends on several very important factors such as the temperature of the atomization flame, the aspiration speed of the nebulizer, the type of solvent and the size of the drops. Generally the atomizers are given in two types these are continuous and discrete. In the continuous atomizer in Llama, the material must be introduced at a constant speed so that the obtained spectrum signal is constant over time, this step is extremely important because it limits the accuracy of the method, that is, a bad atomization process would bring I get errors in the results obtained.
One of the stages of the atomization process is the transport of the solution, where the movement of the solution goes from the container to the nebulizer where it eliminates the solvent material. We must take into account that the process is effective for the material as for the employers, it is advisable that the solutions are equal in their entirety. Then comes the nebulization stage, undoubtedly the most important because as mentioned, the nebulization is responsible for removing all those unwanted particles that can ruin our measurement, its goal is to essentially convert the very fine aerosol solution without any margin of error. So that the flame can be atomized An aerosol with a diameter of 1 to 5μm is needed.
And finally with the atomization process, the last stage has been the equilibration of the vaporized samples , this consists in balancing the vaporized samples where the balance between the oxides and hydroxides, neutral atoms and ions is produced.
Spectrum of atomic absorption AAS
It is easily known or characterized by having resonance lines that have been the result of the electronic transitions of the ground state of the higher energy levels. the spectral lines have a width that is extremely important since this reduces the interference due to the overlapping of the spectra.
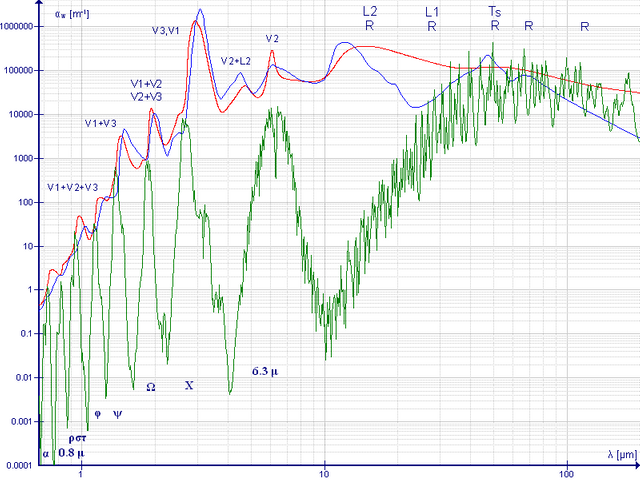 Water absorption spectrum. Absorption coefficient for water - liquid (red line), vapor (green) and ice (blue) between 667 nm and 200 μm (red and most important part of infrared).Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0)
Water absorption spectrum. Absorption coefficient for water - liquid (red line), vapor (green) and ice (blue) between 667 nm and 200 μm (red and most important part of infrared).Attribution-ShareAlike 3.0 Unported (CC BY-SA 3.0)In conclusion is a very complex topic, but little by little you can relate each of my writings, so it is important to start with a theoretical basis when talking about Raman and explain the spectroscopy techniques beginning with the simplest or easiest ones. understand until you reach the most complex.
Atomization absorption spectroscopy covers a lot of field and could easily link 3 more articles related to this topic.
If you want more information about the subject you can visit the following links:
Atomic absorption spectroscopy Atomic Absorption Spectrometry Atomic Absorption Spectroscopy (AAS) Flame Photometry Atomic Absorption Spectroscopy Learning Module Spectroscopy: Interaction of light and matter Atomic spectroscopyPublish through our official app and you will get an extra vote of 5% https://www.steemstem.io/
 Video credits @gtg
Video credits @gtg



This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @utopian-io.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and utopian-io!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 11 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 5 SBD worth and should receive 143 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePigI did a lot of AA for a while when I worked in Pharmaceutical. We had an AA with a Graphite Furnace that used Acetylene fuel and Oxygen. It was pretty fun for a while.
OHHH I feel envy lol I have always wanted to work in one of these. I have only observed how it works in a flame atomizer but I have never been able to use it.
Hi @carloserp-2000!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations @carloserp-2000! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
Click here to view your Board
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard: