Wolbachia pipientis: The World's Most Infectious Microbe?
Wolbachia pipientis, though not as widely known as other disease-causing bacteria, is also quite interesting in itself. I just found out about it recently and after extensive research, I decided to share about this extraordinary microbe.
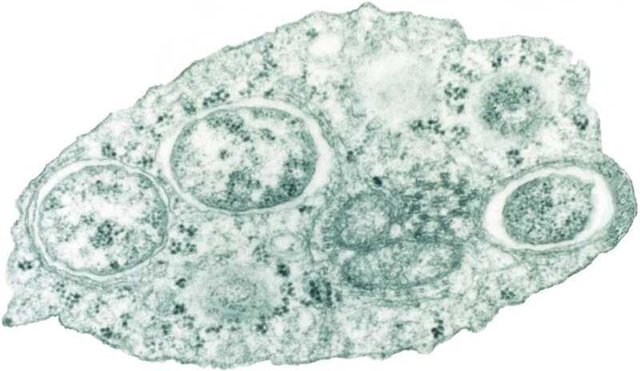
Image Source: Wikimedia CC BY 2.5
It is no coincidence that this bacterium infects more organisms than any other microorganisms. It's been recorded to infect various complexities of organisms like the crustaceans, spiders, millipedes, parasitic worms and mites and may be capable of infecting more than a million insect species around the world.
You may wonder, to what does this bacterium owe its extraordinary success? Simply put, *this endosymbiont is a sensei/master at manipulating its host's reproductive biology and on some rare occasions, has the ability to even change the sex of its host.
W. pipientis lives in the cytoplasm of its host and is transferred from one generation to the next through the eggs of infected females. To maintain its survival, Wolbachia ensures the fertilization and viability of infected eggs while minimizing the likelihood that uninfected eggs survive. However, the mechanism by which this is achieved is dependent on the host.
In mosquitoes and wasps, W. pipientis causes "Cytoplasmic Incompatibility". This is observed when for instance, infected sperm of the wasp Nasonia vitripennis fertilizes infected eggs. Chromosomes from the W. pipientis-laden sperm prematurely try to form an alignment with the egg's chromosomes. These eggs ultimately divide as if they have never been fertilized and develop into males. However, chromosomes behave normally when an infected female mates with an uninfected male. This leads to a normal sex distribution and all descendants are infected with the rickettsia.
When in other infected insects, the W. pipientis may simply kill all the male offsprings and induce parthenogenesis of infected females; that is, the mothers simply clone themselves. This mechanism restricts genetic diversity but gives way to 100% transmission of the rickettsia to the coming generation. Still in some other hosts, the microorganism allows the birth of males but then manipulates their hormone so that the male becomes feminized and produce eggs. This thrilling ability of the microbe to manipulate the reproductive biology of its host has led some to question its potential role in speciation.
This question leads to certain experiments; for instance, two(2) genetically similar North American Wasp species (N. giraulti and N. longicornis) each carry a different strain of Wolbachia pipientis. Predictably, when the two species are allowed to mate with each other, there are no viable offsprings. But when the wasps are first treated with an antibiotic to cure them of their bacterial infections, viable, fertile offsprings are produced. It thus appears that W. pipientis infections, not genetic differences, forms the productive wall that drives speciation. The role of W. pipientis in generating genetic diversity among its host is further demonstrated by the discovery of HGT (Horizontal Gene Transfer) from the bacterium to its host genome. This has been verified in several insect and nematode species that harbor W. pipientis symbiont. In fact, the fruitfly Drosophila ananassae contains the entire W. pipientis genome within its own.
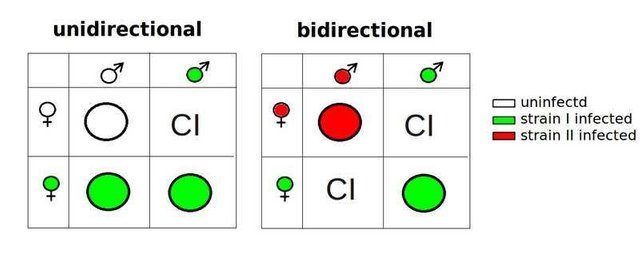
Image Source: Wikimedia Public Domain
Wolbachia pipientis infection has been implicated in several human diseases, most especially, River blindness which has a worldwide incidence of about 18 million people. The causative agent, Onchocerca volvulus, is a filarial nematode transmitted by black flies in Africa and Latin America. When a fly bites a person, the nematode establishes itself in the small nodules beneath the skin, where it can survive for up to 14 years. During this time, it lays millions of larvae, many of which move to the eye. Ultimately, the host mounts an inflammatory response that results in progressive vision loss. It is now recognized that this inflammatory response is principally directed at the bacteria infecting the nematode, not the worm themselves.
Nematode reproduction stops in patients treated with an antibiotic that kills the endosymbiont. While inflammatory damage is irreversible, disease progression is put to a halt. This may prove a more effective and cost-efficient ways of treatment than the current antiparasitic method that must be repeated every six months.
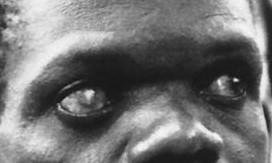
Image Source: Wikimedia CC BY-SA 4.0
Conclusion
Further study of the Wolbachia pipientis' intelligent ways will improve our understanding on how it has evolved to maintain its survival and also continue to grow.
Some scientists have suggested that parthenogenesis may always be attributable to the effect of Wolbachia pipientis.
The microorganism may eventually become a very valuable tool in investigating the complexities of speciation, as well as the solution to curing a devastating disease.
Thanks For Reading, Upvotes, Comments And Resteems are appreciated.
References
Wolbachia pipientis: intracellular infection and pathogenesis in Drosophila.
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Kokooluwa from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.