What do you know about electroplating?
In today's article I want to comment on the usefulness of different materials with metallic coatings and how they are obtained, showing them an example of the deposition of tin on copper through a chemical reduction. This post is an English adaptation of a publication that I made some time ago in Spanish, the Spanish version of this post is available here.
The electrolytic reduction is a means of obtaining active metals, as well as is usually used to purify or refine metals that are in their free state (not combined), to this method of depositing a metal on a surface by the action of electric current also it is known as electrodeposition or electroplating.

Electrodeposition of tin over copper. Own source.
As we know, in our society large quantities of metallic items are consumed, the jewels are covered with gold and silver, electrical contacts are covered with copper, vehicle parts are covered with chrome, as well as sporting goods, just to name a few of the Applications that make electrodeposition one of the most widely used electrochemical processes at the industrial level, not only because of the volume of production but also because of the associated impact, since parts that are generally made up of economic metals acquire better characteristics, such as resistance to corrosion or greater beauty.
So, stay to read to know more about this chemical procedure so useful for the industry.
Electrolysis
It is the process by which electric current is used to force non-spontaneous oxidation-reduction chemical reactions to occur. The reactive system is constituted by a cell and the current enters and leaves from the electrodes, to this type of cell it is known as an electrolytic cell; another type of cell are the so-called voltaic cells. In them, a spontaneous reaction produces electrical energy capable to be supplied to an electrical circuit.
Electrodes
The electrodes are metal surfaces in which oxidation and reduction reactions are produced, and may or may not intervene in these reactions. When they do not intervene, inert electrodes are known; in turn, the electrode in which the chemical species gain electrons, suffering the reduction is called the cathode; while the electrode in which oxidations occur, that is where the chemical species lose electrons , it is called an anode.
Electrodeposition of tin over copper
To demonstrate the electrodeposition process we will use the tin deposition process on copper as an example. To do it we will use a tin wire and a copper coin; we will place the metals in a container with electrolytic solution, the copper coin will be the piece that we are going to cover with a layer of tin, therefore, this piece should adopt the function of the cathode in the cell, for thisit will be connected to the negative terminal of a DC generator. The tin wire will be connected to the positive terminal and will perform the function of the anode. When the circuit closes, the flow of electric current forces the tin to oxidize and passes to the solution in the form of Sn+2 ions; these ions in solution are then reduced to metallic tin at the cathode, being deposited on the copper in the process. As a net result there is no reaction, that is, a conversion of one chemical species into another, but a transfer of tin ions from the anode to the solution, and from this to the cathode, thus achieving the metallic coating of the piece. Semi-reactions of the process would be written as:
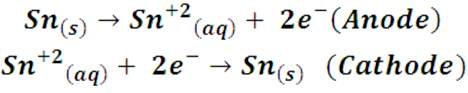
During the process of the cathode (the copper coin) it gains mass when covered with a thin layer of tin, while in tin the loss of mass is observed. These are the results:

Result of electrodeposition: A) coated coin, B) spent tin. Own source.
The tin ions do not pass into the solution spontaneously (that is, this metal does not dissolve when placed in the solution), but when electricity is supplied to the system, the reaction occurs. The electrons are formed at the anode (where oxidation occurs) and are consumed at the cathode; and thus, the source of direct current forces the electrons to move from the positive electrode to the negative, therefore, in all the electrolytic cells the anode is the positive electrode and the cathode the negative.
We will need:

Materials. Own source.
• A beaker or glass container
• 15% HCl solution as an electrolytic medium (I use a commercial cleaning solution, that's why the red color of the liquid)
• A copper coin
• A piece of tin wire
• One 9V battery
• Lead wire
Process:
• The cell will be constituted by the beaker with the HCl solution, we will have to add as much solution as to cover the metals completely.
• We will take the cables and connect them to the terminals of the battery, and then we will connect the tin to the cable connected to the positive terminal, and the copper coin will be attached to the cable connected to the negative terminal.
• Then we will submerge the metals in the solution closing the circuit.
• After one minute we will observe the silver color that the coin has acquired, being able to remove it from the solution and wipe it with water.
• And ready, be surprised with the result.
Watch the procedure in the following video
As you see friends, with this simple experiment we have demonstrated an important industrial application of electrochemistry: electrodeposition. It is certainly necessary to take into account factors such as the type of electrolytic solution and the quantity and time of the supply of electric current, since this will determine very important characteristics of the coating such as the thickness of the metal layer and the quality of its adhesion, but in a way simple we have achieved the effect.
So, that's all for today.I hope to have given you information of your liking and a simple method of giving a silver tone to an implement improving its resistance to corrosion or changing the appearance of a garment or an ornament
Thank you for reading this article, I hope you enjoyed the information. See you in my next post.

References
K. W. Whitten (1985). General Chemistry.
Wikipedia. Electroplating.
Wikipedia. Electrolytic cell.
Chemistry Libre Texts. Electrolysis.
This post has been voted on by the SteemSTEM curation team and voting trail in collaboration with @curie.
If you appreciate the work we are doing then consider voting both projects for witness by selecting stem.witness and curie!
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!