Graphene: A LightScribe Graphene-(LSG)-produced Supercapacitor paving the way for future Portable Electronics
Hello Steemian! It's cool writing on this great platform once again. I'm very glad of being a part of this community promoting quality and original-content post on science, technology, engineering, and mathematics. Today, I'll be doing an exposition on Graphene, a scientific revelation and also on its very important product called LightScribe Graphene-Supercapacitor which is paving the way for future portable electronics.
Introduction
Saying that electricity plays a major role in our lives today is nothing but an understatement because we haven't seen anything yet. Things are changing and technology is evolving every day. In the next few years, all our non-renewable fossil-fuelled vehicles and home-heating will have to change over and start using electric power if we are to prevent global warming because the rate at which our ozone layer is getting depleted gradually is becoming very alarming. Electricity is a type of energy with so many functions. But, despite the immense versatility of electricity, it still suffers one major set back which is the difficulty in storing in a moment.
Batteries, on the other hand, have the ability to store large amounts of electric power but the only problem is that it takes a long time to be fully charged while capacitors charge quickly in a little time but can only store little amounts of power. In the nearest future of electrical and electronics, Supercapacitors will be seriously needed when it comes to storing and giving out large amounts of electricity.

Author: Tavo Romann, Own work, CC BY-SA 4.0
Imagine using a material which is the lightest and best electricity conductor to ever been discovered named Graphene, in producing a supercapacitor, what a revelation that's gonna be. It's going to be massive. So, relax and move along with me as I shed more light on the world of graphene, supercapacitor, and a graphene-made supercapacitor.
So, what is Graphene?
If we are all to agree that the 20th century was the age of plastics, then it's right to say that the 21st century is the age of graphene. Graphene is a two-dimensional material made from sheets of crystalline carbon. It comes in a form of a single and several layers of carbon looking like a honeycomb (hexagonal lattice). It is a recently discovered lightest, thinnest and possibly the strongest and best electrical conductor there ever is. Graphene promises an immense change virtually in everything electronics ranging from computers to cell batteries. Taking a closer look at this picture;

Author: Caleb Roenigk, CC BY-SA 2.0
A pencil like that shown in the picture is a long wood filled with soft graphite. A graphite is an allotrope of carbon that is formed from carbon atoms which are arranged in a place and are held together by weak van der Waals forces. When you use the pencil to write on a sheet, a layer of graphite is the black line that is shown behind. Now, if you can just skim along the surface of a thin layer of graphite, what you would be left with is graphene.
In school, many of us were probably told and we also read in some textbooks (like The New School Chemistry by Dr. Osei Yaw Ababio) that carbon appears in two forms which are called Allotropes. And the two forms are graphite (that soft, black thing inside lead pencils) and diamond (a very hard crystal used as jewelry). The most surprising thing is that these two different materials are composed of the same carbon atoms. Then, why the difference in forms of graphite to diamond. Graphite is dull, black and soft while the diamond is very hard, transparent and sparkling. The difference is none other than in the arrangements of those same carbon atoms in the two materials. The carbon atoms are arranged differently and that's why both graphite and diamond have different properties.
If we are to limit ourselves to what we were taught or read a long time ago, then we are doing ourselves more harm than good because in the last couple of years scientists have discovered numerous other carbon allotropes with even strange and nice properties. Some of which are the fullerenes, nanotubes, and graphene just to name a few.
What is graphene really like?
If you take a time to look inside most of the solid materials known to you, even metals, you would notice some arrangements which are called crystal lattice. In a crystal, there are regular arrangements of atoms in a repetitive form of three-dimension looking like atomic frame climbing each other and having some bonds which are invisible holding them together. Graphite and diamond both exhibit the three-dimensional structure system. Although there's a slight difference in their arrangements. Taking a diamond, for instance, its atoms are firmly bonded together in a 3D tetrahedron. While for a graphite, the atoms are bonded firmly in 2D layers but are attached to the layers above and below by van der Waals forces.
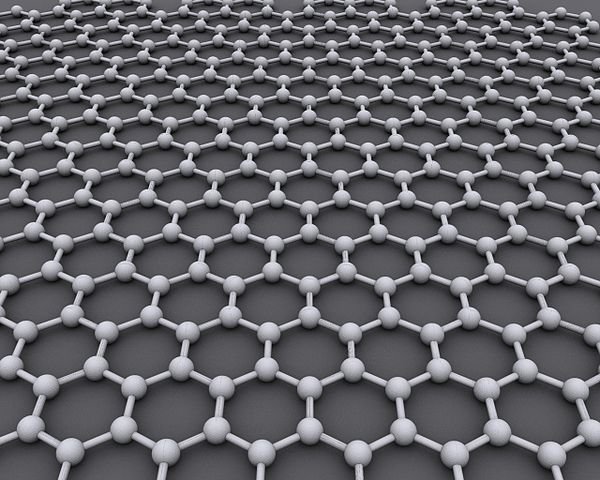
Author: AlexanderAlUS, CC BY-SA 3.0
Graphene is just a single-atom-thick layer of graphite. The extraordinary thing about it is that it appears structurally in two-dimensional. That is, the atoms present in graphene are spread out flat. And just like in a graphite, graphene too has each of its layer made of hexagonal carbon rings but taking a form of a honeycomb. Funny enough, you would need about five million of these layers to make just a millimetre of graphene because the distance between each layer is just an atom high.
New materials are virtually been invented and discovered day in day out, but we rarely hear about them because those inventions or discoveries are not that arousing or attention-grabbing. The discovery of Graphene came about in 2004, but the reason for such excitement about its discovery is because of its immense properties or behaviors. Graphene behaves remarkably well in terms of strongness, stiffness, very transparent and light, and a very wonderful conductor of heat and electricity. Aside from all these beautiful attributes, graphene also possesses extraordinary electronic properties.
First Studies Of Graphene
Although, the first study of graphene was started by Philip Wallace in the year 1947 when he was trying to understand the electronic structure of graphite but the word "graphene" itself was named by Hanns-Peter Boehm, Ralph Setton, and Eberhard Stumpp in 1986. They coined the word graphene from graphite which is an ordered crystalline form of carbon and adding the suffix -ene which is derived from aromatic hydrocarbons like benzene.
In 2004, two physicists from the University of Manchester named Konstantin Novoselov and Andre Geim were able to separate a graphene of a single layer from a graphite by using a simple method known as exfoliation. They placed an adhesive tape on the top layer of a graphite sample and later apply that same layer to another underlying surface material. It was observed that when the tape was later removed, there was an evidence of some graphene left on the underlying surface (substrate) in a single-layer form.
Properties of Graphene
Electronic properties
A very useful property of graphene is that it's a semi-metal with a zero-overlap which makes it a very good conductor of electricity. Normally, in the electronic configuration of carbon, it has two inner atoms and four single atoms in the outermost shell ready for bonding making a total of six atoms. But, each of the four outermost atoms in a graphene is only attached to three other carbon atoms leaving an electron roaming freely in the third-dimensional plane that is ready for electrical conduction. These free mobile electrons are known as pi (π) electrons and are always present below and above the graphene sheet. The pi electrons do intersect and help to improve the C-C bonds in graphene. Basically, the electronic or electrical properties of graphene are decided by the valence and conduction bands of these pi orbitals i.e, the more the bonding of the pi orbitals present, the more the electronic conductivity of the graphene.
Series of tests in time past has made us know that the electronic mobility of graphene is very high and it's between 15,000 cm2V−1s−1 to 200,000 cm2V−1s−1. The electrons of graphene are massless in their movement just like the photons and are able to travel some distance albeit a very small distance without being scattered and the process is referred to as "ballistic transport". By so doing, using silicon dioxide as a substrate, the mobility of graphene is limited to 40,000 cm2V−1s−1.
MECHANICAL STRENGTH
In terms of mechanical strength, graphene also excelled. It is the strongest material that's ever discovered. Each of its carbon bonds is 0.142Nm long. Graphene boasts a tensile strength of 1.3×1011pa (130 gigapascals), a far better mechanical strength than structural steel with 4×108pa (0.4 gigapascals) and Kevlar having a strength of 3.75×108 (0.375 gigapascals). Aside from the fact that graphene is the strongest, it's also one of the lightest material at 0.77milligrams per square metre. To understand its lightness better, it means a square meter of paper is approximately 1000 times heavier than graphene. It is mostly reported that a single sheet of graphene that will be enough to cover an entire football pitch would just weigh 1 single gram.
Author: Mariolan22, CC BY-SA 4.0
Another interesting thing that really makes graphene to be so special is in its elastic properties. It has the ability to return to its original length after strain and no matter the deformation. In 2007, some tests like the AFM (Atomic force microscopic) were carried out on graphene and it was observed that graphene sheets with thickness that varied between 2 and 8 Nm had elastic constant between 1 to 5 N/m and a stress to strain ratio of 0.5×1012pa (0.5 terapascals). All these figures show that graphene has a very good elastic property.
OPTICAL PROPERTIES
Finally, graphene also has very beautiful optical properties. It has the rare ability to absorb a large amount of white light up to 2.3%. This is an interesting property considering the fact that graphene is very small with a thickness like that of an atom. It has the ability to absorb such a large amount of white light because of its electronic properties (as a result of its massless charge carriers having a very high mobility) which has been explained above. In a research conducted a few years ago, it was confirmed that the amount of white light that can be absorbed by graphene is actually founded on the Fine Structure Constant (FSC), rather than being controlled by details of the material. It was also noticed that adding another stratum of graphene didn't decrease the rate at which the white light is absorbed but rather it was increased by the same value of 2.3%. The Opacity of Graphene (πα) which is approx. 2.3% is equal to a generally accepted conductivity value of G=e2pa/4ℏ (±2-3%) over the range of frequency where the white light can be visible
LightScribe Graphene-(LSG)-Supercapacitor
Supercapacitors or ultracapacitors popularly known as Electrochemical capacitors (ECs) are really different from the normal capacitors you see in your computer monitor or television in that they have the ability to store a very large amount of charges. ECs have been well known as a good device for storing energy because of their ability to charge and discharge faster than most batteries, but are still hindered by low energy densities as compared to batteries. To produce an EC that can both store a large amount of charges and also have a relatively high energy density as that of batteries would really represent a great technological advancement in the storage of energy. And to be able to achieve this great feat, one needs a new type of electrode that can sustain high conductivity and that can also provide higher surface area than the ordinary ECs that are known to use activated charcoal electrodes.
Presently, some researchers at the University of California, Los Angeles have used a standard LightScribe to produce such electrodes. Before we go in deep, let me quickly tell you what is meant by LightScribe:
It is a new technology of optically recording disc. It uses some rare types of coated recordable CDs and DVDs media to make text or graphics labels using a laser which is different to printable and label-sticked discs. source

Author: Rico Shen, CC BY-SA 3.0
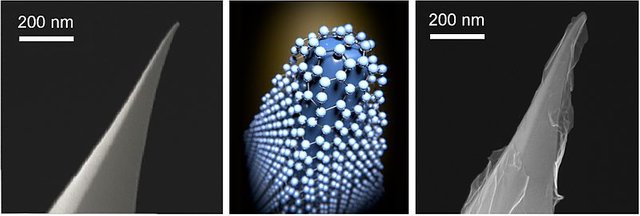
The electrodes are made up of an embedded network of an atom-thick graphene that exhibits great mechanical properties, electrical properties, and high surface area.
The researchers were able to produce a graphene-based supercapacitor that's highly efficient with excellent electrochemical features under high tensile stress. They were able to produce such a strong and efficient material by covering a DVD disc with a thin film of graphite oxide which is then treated by laser inside a LightScribe DVD to make graphene electrodes.
As expected, energy storage devices are judged by two important qualities which are its power density and the energy density. Let's assume we are using the energy storage device to power an electric car, the energy density will show us how fast the far can travel in a single charge while the power density will only let us know how fast the car can run. But, the device made in UCLA with Laser Scribed Graphene (LSG) electrodes have very high energy density values in various electrolytes and still maintain the high power density, thereby packing two great features in one.
“From our study, it was shown that the new graphene-based supercapacitors can store as much charge as normal batteries, and can be charged and discharged a hundred times faster,”
-Richard B. Kaner, Professor of Chemistry & Materials Science and Engineering.
“From here at UCLA, we displayed a platform for the making of highly-performance graphene-based ECs from a solid-state method that avoids the re-piling of graphene sheets over each other,”
-Maher F. El-Kady, the lead author of the study and a graduate student in Kaner's lab.
LSG produced electrodes are very high in conductivity. They have approximately 1700 S/m as compared to activated charcoals which have between 10 to 100 S/m. What this means is that LSG electrodes can be exactly used as supercapacitor electrodes without even having the need for current collectors as is the case for normally activated charcoal (carbon). Finally, these attributes allow LSG to behave as both the active material and current collector inside the Electrochemical Capacitor. Combining the two functions in a layer paves the way for a simple design and it also makes LSG supercapacitors very economical to purchase.

Author:Liu L, Yu Y, Yan C, Li K, Zheng Z; CC BY-SA 4.0
The future applications of LSG-based supercapacitors
Owing to the fact that LSG-based supercapacitors are very light in weight, low cost of production, great elastic and mechanical properties, it is very likely in the next few years to see technology starting to use them. Also with more development in energy storage system, LSG supercapacitor will be needed in many different applications.
Motor vehicles that use supercapacitor are already widespread in the society. A Chinese company is presently producing buses that works on supercapacitor energy system like those used in Formula One racing cars. His buses are made in such a way as to be able to store energy whenever a brake is applied and later use the energy stored to power the bus till the next bus stop.
Furthermore, there will come a time probably in the next couple of years where our mobile phones and electronics are being powered by a supercapacitor. They won't only be able to be charged at a very higher rate than the current lithium-ion batteries but also have the ability to last for a very long time.
Supercapacitors can also be used as a power back up for our homes and industries. Also, if you have a vehicle using fuel cells that are able to store a large amount of energy, conversely can also be used to power your home when there's power failure at home.
While LSG-based supercapacitors are presently a possible solution in the future, technology needs to be moving at a high speed so as to make it come true. But, let's not be dismayed and keep our fingers crossed since many companies of the world are already laying down the plan for this technology to come into place and employing new methods of reducing the rate at which fossil fuels are being used.
Thanks for reading.
References
Fundamental properties of graphene
Graphene; a simple introduction
What is graphene?
Graphene; its chemistry
Researchers develop LSG at UCLA
How do Supercapacitors work?
Graphene Supercapacitors, what are they?
What is LightScribe?
Graphene, one material that has got 1000 uses.
Very immense and vast in its uses. Its potentials haven't yet been fully utilized.
@greenrun, thanks for passing by.
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!
Resteemed your article. This article was resteemed because you are part of the New Steemians project. You can learn more about it here: https://steemit.com/introduceyourself/@gaman/new-steemians-project-launch
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by emperorhassy from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.
Congratulations! Your post has been selected as a daily Steemit truffle! It is listed on rank 18 of all contributions awarded today. You can find the TOP DAILY TRUFFLE PICKS HERE.
I upvoted your contribution because to my mind your post is at least 16 SBD worth and should receive 208 votes. It's now up to the lovely Steemit community to make this come true.
I am
TrufflePig, an Artificial Intelligence Bot that helps minnows and content curators using Machine Learning. If you are curious how I select content, you can find an explanation here!Have a nice day and sincerely yours,

TrufflePig