THE DISCOVERY OF RADIOACTIVITY
 )
)Becquerel decided to test his assumption. He put crystals of a uranium salt on top of a photographic film, which was sealed in black paper that kept out all light. He let the Sun shine on the crystals and, as he expected, when the film was developed, it had become fogged.
On 26 February 1896, clouds shut out the Sun, so Becquerel just put the crystals, unexposed to sunlight, in a drawer on top of some sealed photographic film. Three days later, he decided to develop the film anyway, just to check that there was no image. To his amazement, he found a clear area of darkening on the film below the crystals. He concluded that some unknown kind of radiation that is not dependent on the Sun and that could pass through the black paper must have come from the crystals. It took the work of others to discover more about this radiation.
With her husband Pierre, Marie Curie began to study this phenomenon. She investigated other uranium compounds and found that they, too, gave off this new radiation. She called it radioactivity. Radioactivity is the spontaneous break-up of atomic nuclei, giving out rays called radiation.
The Curies observed that pitchblende, an ore known to contain uranium, was much more radioactive than they expected from the uranium in the ore alone. The Curies later discovered two new radioactive elements, radium and polonium. It was radium that gave pitchblende its high radioactivity. The radiation of radium had so much energy that it burned the skin, and while uranium fogged film after a few hours, radium did so instantly. Marie Curie also discovered another radioactive element, thorium.
 )
) )
)Marie Curie (1867-1934) was the first person to receive two Nobel prizes. The first was for physics (1903), which she shared with her husband and Becquerel for work on radioactivity. The second was for chemistry (1911) for her discovery of radium and polonium (named after her homeland, Poland). At the time of her discoveries it was not realized that the radiation to which she was exposed would cause the leukemia that was to end her life.
THE THREE RADIATIONS
Radioactivity is the spontaneous breakdown (disintegration) of a nucleus and the emission of radiation. 'Spontaneous' means something that happens without anything seeming to cause it.Some naturally occurring isotopes are unstable and will break down. As a result, they release at least one of the three principal types of radiation which are the alpha (α), beta (β), and gamma (γ) radiations. All three can knock electrons off atoms they collide with, and so they are called ionizing radiations.
NUCLEAR EQUATIONS AND RADIOACTIVE DECAY
Alpha decay
Any atom with an atomic number greater than 82 will be radioactive, and most of these elements decay by emitting alpha radiation.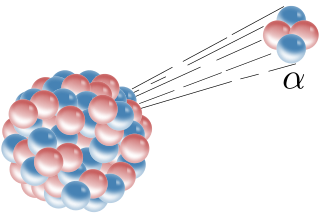 )
)24995Am ― 23793Np + 42He
i.e Americium breaks down to Neptunium and an alpha particle.
By studying the equation above carefully, it can be seen that mass numbers and atomic numbers need to balance on each side of the equation. There are two other points to note:
1. In alpha decay equations, we do not write He2+. This is because the alpha particle comes from the nucleus of a larger atom - it is not a helium atom which has lost electrons. However, alpha particle do eventually gain electrons and become helium atoms. 2. In nuclear equations, we always write the atomic number, even though we know the name of the element.Now let’s look closely at what happens to americium. It becomes a new element because its atomic number decreases by 2. The event of one element changing into another in this way is called a transmutation.
Beta decay
Some isotopes of lighter elements have too many neutrons and are unstable. Such isotopes decay by emitting a beta particle (a high-speed electron). When a nucleus releases a beta particle, a neutron changes to a proton, and this makes the atom of the new element more stable.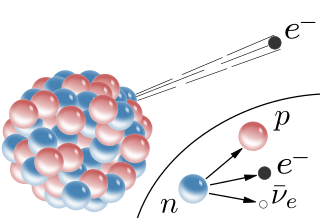 )
)The next nuclear equation is the equation for beta decay. note that in this equation, the electron is given values of zero and -1 i.e;
10n ― 11p + 0-1e (0-1e is a beta particle)
The nuclear reaction of carbon-14 is a good example of beta decay, and beta particles are detected in radiocarbon dating:
146C ― 147N + 0-1e
Since a neutron changes into a proton, the atomic number of the new element is increased by one, but the mass number stays the same.
Gamma rays
Gamma rays arise when the nucleus has excess energy i.e when it is in an excited state. The nucleus is usually excited after a nuclear decay, for instance after releasing alpha or beta particles. It loses its excess energy by emitting gamma rays, which are very high-energy electromagnetic radiation. Gamma radiation on its own does not result in the formation of a new element. )
) )
)HALF-LIFE AND THE RATE OF RADIOACTIVE DECAY
Radioactive isotopes never stop decaying and, unlike chemical reactions, their rate of decay is not affected by pressure and temperature. The rate of decay of a radioactive isotope is measured as the half life, t1/2 which is the time it takes for half of the isotope to decay, and is unique to every isotope. The most stable are those with the longest half-lives, and the least stable have the shortest half-lives. For this reason, many of the very unstable isotopes which can be artificially synthesized do not exist in nature.
Iodine-131 is a very unstable isotope. Half of it will decay in 8.1 day (t1/2 = 8.1 days). It is useful in medical diagnosis, to detect liver and brain tumours and to investigate the human thyroid gland.
Cobalt-60 is a much more stable isotope. It takes more than five years for cobalt to decay to half its original mass. Its beta and gamma rays are used to sterilize medical equipment and to attack cancer cells in the human body.
 )
)At the other end of the scale, uranium-238 was formed before the Solar System existed, and takes 4.5 × 109 years for half of it to decay. When atomic weapons were tested in the 1950s and 1960s, the isotope strontium-90 was released into the Earth's atmosphere for the first time, and was deposited throughout the world. It entered the human food chain through water supplies and, in particular, through milk from cows grazing on contaminated pastures. Since strontium is chemically very similar to calcium it becomes incorporated into people's teeth and bones, and is linked to an increased risk of developing leukemia and bone cancer.
Strontium-90 has a half-life of 28 years, so starting with 20 mg, 28 years later half will have decayed, leaving 10 mg of strontium-90. In a further 28 years there will be only 5 mg. After 84 years, which is three half-lives, only 2.5 mg will remain, and so on. No matter how much strontium-90 there is to begin with, half disintegrates every 28 years. The picture below is a graph of this decay known as exponential decay, and its shape is typical of all radioactive isotopes.
 )
)DATING AND OTHER USES OF RADIOACTIVITY
It is because radioactive isotopes have fixed half-lives, which temperature or pressure cannot alter, that we can use half-lives to date objects, and even date the Earth itself. We also use radioisotopes elsewhere, particularly in medicine.
Carbon-14 dating
The best-known radioactive dating technique uses carbon-14, which has a half life of 5730 years. The Earth is continually bombarded by particles from the cosmos. These 'cosmic rays' crash into atoms in the upper atmosphere and cause them to release high-energy neutrons. They, in turn, smash into the nuclei of nitrogen-14 atoms in the upper atmosphere, and produce carbon-14 which is radioactive.147N + 10n ― 146C + 11H
Carbon-14 reacts with oxygen to form 14CO2, and this is used by plants in photosynthesis to make carbohydrates. Through food chains, the isotope becomes incorporated into all living things, including ourselves. As soon as the carbon-14 is produced it starts to decay by emitting beta particles:
146C ― 147N + 0-1e
The amount of carbon-14 in the atmosphere stays roughly constant because, over time, the rate of decay of carbon-14, and its rate of production by cosmic rays have become balanced. It is important to realize that the amount of carbon-14 produced each year is very small indeed –only about 7.5 kg in total – and the ratio of carbon-14 to carbon-12 all around us and inside us is also very small. There are 12 million million carbon-12 atoms for every one carbon-14 atom. This ratio of carbon-12 to carbon-14 in any organism stays constant while it is alive, because any carbon-14 that decays is replaced in the turnover of food and nutrients from its environment. When the organism dies, it no longer takes in carbon-14, so the amount of carbon-14 starts to decrease.
 )
) )
)Imagine that archaeologists find a wooden carving they think is thousands of years old. They find that its carbon-12 to carbon-14 ratio is 24 million million to 1 – that is, the carving has half the amount of carbon-14 that is found in wood cut down today. The carving is therefore one half-life old, or about 5730 years.
THE USEFULNESS OF RADIOACTIVITY
Radioactive materials are being used in an increasing number of ways. Some of which are in:
Treating cancer
Cancerous cells are abnormal cells that divide at a rapid rate, producing tumours that invade surrounding tissues. Some types of radiation can cause a cancer. At the same time, cancerous cells are easily destroyed by carefully controlled doses of radiation, which do not affect other surrounding cells. Over a period of weeks, a tumour in a patient can be made inactive by exposure to doses of gamma rays. The radioisotope generally used for this treatment is cobalt-60.Tracers for diagnosis and treatment
A radioisotope has the same chemical properties as any other atom of the same element, and molecules of a substance ‘labelled’ with a radioisotope can therefore be traced because their radiation can be detected. For example, iron-59 can be introduced into haemoglobin to follow the production of red blood cells in bone marrow, lodine-131 as a label in sodium iodide (Na231I) is used to investigate the activity of the thyroid gland and to diagnose and treat diseases of this gland.Labelled iodine also helps to detect liver and brain tumours. Sodium-24 as sodium chloride (24NaCl) solution is put into the blood to follow blood flow and locate clots and obstructions in vessels. Tracers must have a half-life that is long enough to allow detection but short enough not to cause undue damage to body cells.
Tracers in agriculture
Labelled sodium iodide in boreholes indicates the route taken by water underground. Barium sulphate, which is insoluble in water, can be labelled with barium-140, and the labelled compound 140BaSO4 is used to monitor how silt is deposited in rivers. Phosphorus-32 in phosphate compounds helps to trace the uptake of fertilizers by plants.Dating the Turin shroud
This has been one of the most famous uses of radiocarbon dating. The Shroud of Turin is a linen cloth, over 4 metres long, bearing two images of a man - one of the front and the other of the back of someone who appears to have been crucified. For centuries many people have believed that the cloth wrapped the body of Jesus after his death. According to the first reliable records, the cloth appeared in France in 1350, and was later taken to the Italian city of Turin.In recent years, chemical tests were done on small samples of the cloth. They revealed that the image was not painted on to the cloth by any known method, and that the blood stains are definitely human.
In 1988, laboratories in Oxford, Zurich and Arizona were each given pieces 2 centimetres square to date independently. The linen was found to have come from flax grown somewhere between 1260 and 1390. For the cloth to have been the burial shroud of Jesus, the flax would have had to have grown some time before his death. But some people still claim that a burst of energy during the resurrection could have made the flax seem younger than it is. Many questions remain unanswered, including the way that the image was formed.
Finally, is Radioactivity safe or not?
Radioactive materials have proved themselves useful to society, especially in medicine. Yet we often hear that exposure to radiation is dangerous. Is there a contradiction?We have looked at three types of radiation - alpha, beta and gamma - which come from the breakdown of unstable nuclei. These are called ionizing radiation because when they pass through molecules they can knock off electrons and form ions. This disrupts molecules. In living matter, many of the molecules are complex and contain weak bonds. DNA, the carrier of genetic information, is an example. So exposure to ionizing radiation can alter or destroy important biomolecules, and can result in illness and even death.
 )
)Whether ionizing radiation causes damage depends on two properties: the penetrating power of the radiation and its ability to ionize (and so disrupt) molecules. Alpha particles are relatively massive, so can be very damaging, but their penetrating power is very low, so they do not progress far in living tissue. Beta particles penetrate further, but they are lighter and so cause less ionization of molecules. Gamma radiation is the most penetrating of the three, but because it has no mass or charge, it is the least ionizing.
In using radioisotopes, particularly in medicine, we balance the risk of the damage that a form of radiation may do to biomolecules with the benefits that using it can bring. We are exposed to natural radiation all the time and our own molecules contain radioisotopes, but we also have mechanisms to repair radiation damage. We cannot eliminate exposure altogether, but we can minimize and control it.
I'm always fascinated by the history behind the discovery of most of the scientific methods that makes life so better today. Thanks for sharing.
Thanks, @greenrun. I'm glad you came around. It's been awhile.
I'm always fascinated by the history behind the discovery of most of the scientific methods that makes life so better today. Thanks for sharing.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Thanks for having used the steemstem.io app. You got a stronger support!
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPDo not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!
Hi @empressteemah!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV