The History of Mendeleev and the development of the Periodic Table.

Pixabay
Talking about modern day alchemy and the discovery of 'super-heavy' elements: Ever since Mendeleev proposed his theory of the periodic classification of elements, scientists have set themselves the task of discovering yet more elements. During the past 40 years or so, the search has been on to discover elements that are not normally found on Earth. Scientists have bombarded atoms with high-energy particles in an effort to achieve the alchemist's dream of transmutation, the changing of one element into another.
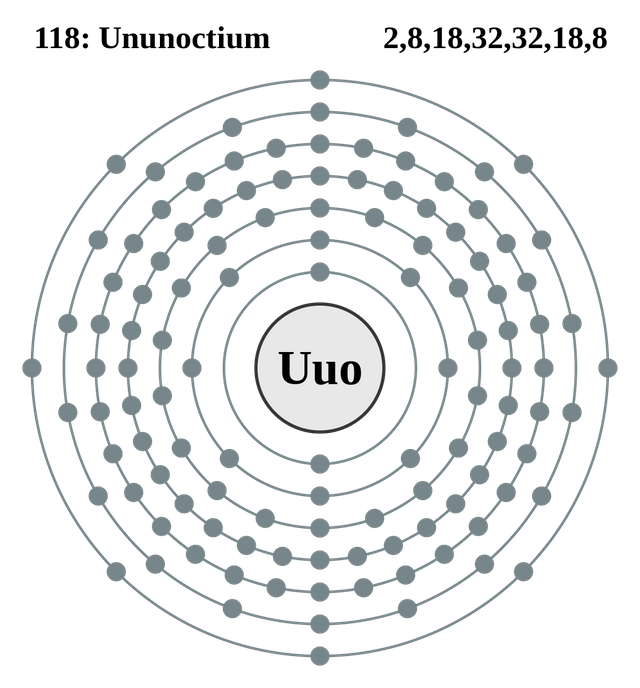
In 1999 scientists in California claimed to have made the 'super-heavy element' atomic number 118. They predicted from its position in the Periodic Table that the element would be the seventh noble gas. Three years later these scientists retracted their discovery, because they were unable to repeat their findings. However between 2002 and 2005, collaborative research involving Russian and American scientists continued, and eventually sufficient evidence was collected to announce the discovery of element number 118. The scientists' results indicated that they had made just a few atoms of this new element, which then spontaneously broke down into simpler elements.
The element was given the symbol Uuo and the name ununoctium. It may be many years before scientists can agree to give it a two letter atomic symbol and a proper name, because there has to be international agreement about the names of elements.
Wikimedia, CC BY-SA 2.0 uk
DMITRI MENDELEEV AND HIS PROPERTIES OF ELEMENTS.
For thousands of years, people have been curious about the nature of materials in the world around them, and have had theories on the simplest forms of matter, or 'elements', that make up these materials.
However, only in the past 200 years, since modern experimental work began, have chemists been sure that they have identified elements - substances that could not chemically be changed to anything simpler. Only then could the chemists start to devise useful theories on how elements are related to each other, theories that would help scientists progress in their knowledge and understanding of materials.
The greatest breakthrough in this recent endeavour was presented to the world of chemistry on 17 February 1869, when the Russian chemist, Dmitri Mendeleev, published his work on the properties of elements. Assembling the observations and discoveries of earlier workers, he had assembled the symbols of the chemical elements in the order of their atomic masses. Mendeleev's particular arrangement became the first modern Periodic Table and was to prove invaluable to chemists. It greatly accelerated the discovery of new elements and understanding of their properties, and enabled scientists to predict the lull range of elements that were eventually found or artificially made.
This post serves to describe how the Periodic Table developed. I'll also explore its importance, both in explaining chemical trends in the elements, long before the structure of the atom was understood, and also in predicting the discovery of unknown elements.
EARLY IDEAS ABOUT THE ELEMENTS
More than 2000 years ago the Ancient Greeks pictured all the material in the world being composed of four 'elements' - earth, water, air and fire. The idea may seem far fetched now, but the Ancient Greeks were thinkers and not experimenters. It is interesting, however, that their model reflected the three states of matter. as solid, liquid and gas - and fire, which has the capacity to transform materials.
Civilisations, some of them older than the Ancient Greeks, were able to produce metallic elements such as copper and mercury by mixing their ores with charcoal and heating them in a simple furnace. Other elements, including gold and silver, were found naturally in their
elemental state. The drive to produce metals and their alloys arose not because people were interested in chemistry for its own sake, but because of the need to make tools and weapons that were hard-wearing and shatterproof.
The early Arab civilisation was noted for its practical interest in chemicals, and has given us words currently used in chemistry such as alchemy and alkali. In the Middle Ages, particularly, alchemists in Europe tried hard to find a way to make gold from lead and other metals, not knowing that this was chemically impossible.

Pixabay
When the pace of discovery of the elements was shown on a graph, it was observed that the graph was nearly vertical implying that several elements were discovered at the same time. Typically, this happened when a new theory or idea was proposed, or new apparatus was developed.
For example, towards the end of the 18th century, glassware designed for gas preparation led to the discovery of oxygen, chlorine and hydrogen, and air was shown to be a mixture of gases, not a single substance. Later, the new technique of electrolysis enabled Humphry Davy to discover another group of elements - sodium, potassium, magnesium, barium and strontium.
DALTON'S ATOMIC THEORY
In Dalton's atomic theory, he proposed that an atom was the smallest part of an element and could not be split, and that atoms of a particular element had a characteristic mass. We now call this mass the element's relative atomic mass, but in Dalton's time it was known as the atomic weight.
Through the early 19th century, chemists continued their search for new elements, gradually improving their techniques, but working in an unsystematic way, as they lacked a basis for predicting the existence of unknown elements.
DÖBEREINER AND HIS TRIADS
Johann Döbereiner was among the scientists who were looking for ways to classify the elements. In 1829, he reported on patterns he had found among the elements known at the time. He noticed that elements with similar properties could be grouped into threes, or triads, and that there was a mathematical pattern in the values for their relative atomic masses.
One of his triads was lithium, sodium and potassium, elements that Davy had discovered using electrolysis.
NEWLANDS' OCTAVES
Döbereiner had begun to bring order to the elements., showing patterns that existed in their relative atomic masses. Then, in 1864, John Newlands arranged the elements in the order of their relative atomic mass. He, too, found a pattern, noticing that elements with similar chemical properties were eight positions away from each other, rather like the notes in a musical octave. (At this time, the noble gases, which would have added another position, had not been discovered.)
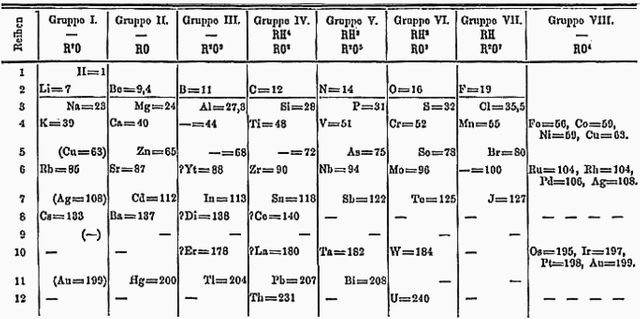
Looking at the table below. If you start with lithium as '1' and count eight elements, you come to sodium. The two elements are very similar physically and chemically. Count another eight elements and you reach potassium, also very similar to lithium and sodium.
| Newlands' octaves | ||||||
|---|---|---|---|---|---|---|
| hydrogen | lithium | beryllium | boron | carbon | nitrogen | oxygen |
| fluorine | sodium | magnesium | aluminium | silicon | phosphorus | sulphur |
| chlorine | potassium | calcium | chromium | titanium | manganese | iron |
Though the rule of octaves works for some elements, there are exceptions. Look at sulphur and iron, which are, again, eight elements apart and yet have very different properties. It needed another system of grouping to make sense of these differences, but Newlands was too preoccupied with the idea of an octave to devise an explanation.
MENDELEEV AND THE DEVELOPMENT OF THE PERIODIC TABLE
The Russian chemist Mendeleev was fascinated by the elements. He wrote down each known element and its properties on a separate card, and began to put them in a logical order as is shown in the table below. He ignored hydrogen, for it did not seem to fit anywhere, and started with lithium. Guided by atomic weights, he put beryllium, boron, carbon, nitrogen, oxygen and fluorine in a column. The next element was sodium. He put it next to lithium as both were reactive soft metals. Then the elements magnesium, aluminium, silicon, phosphorus, sulphur and chlorine duly took their place.
Mendeleev continued his list with potassium and calcium. The obvious place for the next card, for titanium, seemed to be below calcium. Then Mendeleev made a brilliant decision. He recognised that a gap had to be left between calcium and titanium for an element that was yet to be discovered.
The chemical properties of titanium were more like those of carbon and silicon than of boron and aluminium, and so Mendeleev placed titanium next to silicon. Mendeleev concluded, "the elements, if arranged according to their atomic weights [now known as relative atomic masses], exhibit an evident periodicity of properties."
| Mendeleev's early lists of elements | ||
|---|---|---|
| lithium | sodium | potassium |
| beryllium | magnesium | calcium |
| boron | aluminium | ? |
| carbon | silicon | titanium |
| nitrogen | phosphorus | vanadium |
| oxygen | sulphur | |
| fluorine | chlorine |
Notice that Mendeleev first placed the elements in columns. Soon afterwards, he decided on the arrangement of the Periodic Table that is familiar to us. Each element had its own number and fixed position in the table, and Mendeleev left several gaps for elements that were then unknown, but which he believed existed.
Den fjättrade ankan at Swedish Wikipedia. • Public domain
Mendeleev's table made a considerable impact among chemists of his time, because it was so useful to them. It revealed clear patterns and trends in the properties of elements, both known and unknown. For the first time, chemists could guess at the total number of elements that might exist and, in noting the gaps, they were spurred on to make discoveries of new elements.
Mendeleev used his table to predict the properties of scandium and gallium, and also germanium, which he first named 'eka-silicon'. Within 20 years, all three elements had been discovered. The Table below shows the properties he expected germanium to have, and how close his predictions proved to be.
Altogether, Mendeleev predicted properties for ten unknown elements, and was later proved correct in eight cases.
A table showing the properties of 'eka-silicon', germanium and tin.
| Silicon | Predicted properties for eka-silicon | Actual values for germanium (eka-silicon) | Tin | |
|---|---|---|---|---|
| Atomic mass | 28 | 72 | 72.59 | 118 |
| Density /gcm-3 | 2.3 | 5.5 | 5.3 | 7.3 |
| Appearance | grey non-metal | grey metal | grey metal | white metal |
| Formula of oxide | SiO2 | EkO2 | GeO2 | SnO2 |
| Formula of chloride | SiCl4 | EkCl4 | GeCl4 | SnCl4 |
| Reaction with acid | none | very slow | slow with conc. acid | slow |
Mendeleev positioned elements on the basis of their properties. Where elements did not fit in order of their atomic weights (relative atomic masses), he assumed that these had been determined incorrectly. So, for example, he placed cobalt before nickel, though cobalt has greater mass.
ATOMIC (PROTON) NUMBER
We have seen that, on the basis of their properties, a few elements in the Periodic Table had to be placed out of sequence in terms of their relative atomic masses. We now know that relative atomic mass depends on the overall structure of the nucleus, and that it is the order of atomic number of elements that determines the trends in their properties.
The atomic number (or proton number) of an element is the number of protons found in one atom of that element.
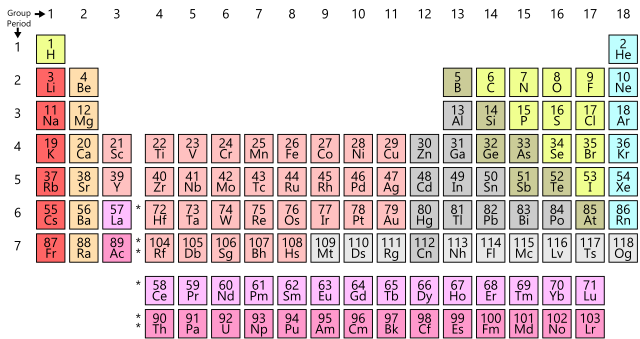
PERIODS AND GROUPS IN THE PERIODIC TABLE
The table Mendeleev devised in 1905 has much in common with a modern version of the Periodic Table. Horizontal rows of elements represent periods, numbered 1 to 7. Note how the seven modern periods compare to Mendeleev's series, and how they differ.
The vertical columns 1 to 7 and 0 represent the groups of elements. Elements in a group have very similar chemical properties. Notice that each group has two columns. These correspond to a group in the s and p block and another group taken from the d-block elements. We just refer to Group 1 being all the elements in the vertical column headed by lithium, and similarly, Group 7 is all the elements headed by fluorine.
Wikimedia, Offnfopt • Public domain
References
Historical Development of Periodic Table: Mendeleev, Doberiener
History of the periodic table - Wikipedia
Development of the Periodic Table - Chemistry LibreTexts
Development of the periodic table — Science Learning Hub
Dmitri Mendeleev and a brief history of the periodic table - SpringerOpen blog
Development of the periodic table - Royal Society of Chemistry
About Dimitri Mendeleev - Periodic Table
Dmitri Mendeleev - Wikipedia
Dmitri Mendeleev | Biography & Facts | Britannica.com
An interesting article.
I remember writing something for junior high school students about Mendeleev. I was impressed then by how broad his field of interest was, not only in chemistry but in other intellectual endeavors. He was snubbed by the Nobel Committee and never did receive the well-deserved prize. Still, we all recognize him and every junior high school student knows his name.
Thanks for coming by, @agmoore2.
I first got to know about Mendeleev in the senior high school and since then I've been fascinated with all his great works. But, It's a pity he wasn't given a Nobel prize for all his efforts.
That's when I first heard about him also. Then when I became a teacher, I had to hit the books again. Have to be ready for those questions that come out of nowhere :)
Thanks a lot about this post. I always enjoy reading about the history of science (and I learned here a few things, my chemistry being far far far behind me ;) ). I am looking forward to read more from you.
It's a pleasure, Prof. Thanks for reading the post. I'm glad you came by. It's been a while I last saw your comments.
Yeah @lemouth, you can be on the look-out for more chemistry and biochemistry posts.
I was quite away for the last two months, as mentioned in the post I wrote yesterday. This is the reason why I didn't comment much recently ;)
Fun fact, I remember having written on Steem about the confirmation of the element 118 two years ago (together with the elements 113, 115 and 117 that were confirmed at the same moment). You can check here if interested ;)
I later saw the post where you stated the reasons u've been absent for a while. Studying science and conducting researches are two great things, but so also is your health and mental wellbeing. Take good care of yourself and always stay healthy.
Fun check, lol, I just finished reading the post you wrote on the confirmation of elements 113, 115, 117 & 118. It's an awesome post. Thanks for the share.
You are very welcome!
It's a pleasure, Prof. Thanks for reading the post. I'm glad you came by. It's been a while I last saw your comments.
Yeah @lemouth, you can be on the look-out for more chemistry and biochemistry posts.
A good historical exemplar of how theory spurs practical discovery, and also makes clear predictions which makes it very testable and therefore very scientific in a Popperian way.
Thanks for the beautiful comments, @alexander.alexis. I'm glad you came by to read the post.
Hi, thanks for the post! The entire history was a good read, but I thought it was especially interesting where you pointed out how the gaps in the newly created table spurred discovery of the missing elements.
I included a link to your post in my recent article, Interesting Links: June 6, 2019, and set the beneficiaries so that you'll receive 5% of that post's rewards.
Wow..... Thanks, @remlaps-lite for the kindly gesture. This is so great. I'm also glad you came by to read the post and equally found it interesting. I really do appreciate.
Mendeleev's contribution to science particularly chemistry is a tremendous one. Good job here.
Yes, u're absolutely right - a very immense contribution to chemistry.
@greenrun, thanks for the good comment and also for coming by.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Please consider using the steemstem.io app to get a stronger support.
Hi @empressteemah!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!