THE WORLD OF POLYMERS.
INTRODUCTION
Most polymers that have revolutionised our everyday living are manufactured from chemicals that come from non-renewable crude oil. As the reserves of this precious resource are used up, the hunt is on for other sources of polymers, and growing crops that provide them is one promising option. We are all familiar with the natural polymers: starch, cellulose, protein and DNA; certain bacteria produce other polymers that are the subject of intense research throughout the world.
Pixabay
Biopol, a polyester, was the world’s first bacterial polymer to be produced commercially. The polymer makes up 80 per cent of the dry weight of the bacterium Alcaligenes eutrophus, which uses it as an energy store in the same way that we use fat. The first articles made of Biopol, bottles for hair-care products, appeared in 1990 and it found uses in coating paper bags and in producing disposable cutlery. However bacterial polymers are much more costly to produce than synthetic polymers from crude oil. This meant that it did not take off in the way the manufacturer had hoped and production virtually ceased. But another way to make this plastic was discovered.
We waste millions of tonnes of food each year from canteens and restaurants. Most of it ends up in landfill sites and produces the potent greenhouse gas, methane. Researchers in Hawaii have used bacteria to convert this waste food into Biopol, also known as BHA, and other plastics, depending on the conditions used. For every 100 kg of waste food slurry, 25 kg of polymer can be produced. This has created renewed interest in polymers made by bacteria.
The outstanding advantage of bacterial polymers is that they are totally biodegradable. In a world in which the disposal of synthetic polymers is reaching crisis levels, as landfill sites become scarce and the incineration of plastic waste adds to atmospheric pollution, this is a very important virtue. Even if it is incinerated the only combustion products are carbon dioxide and water, and it releases more energy in the process than its synthetic counterparts.
SO, WHAT IS A POLYMER?
More chemists work with polymers than with any other type of material. Polymers enter every aspect of our lives. The DNA that carries the genetic code is a natural polymer, as are proteins, starch and cellulose. Chemists started to make synthetic polymers in the early 1900s and now we are dependent on them. They are used in clothing, packaging, furniture, adhesives, inks, coatings and electrical equipment, to mention just a few.
Polymers are very large molecules with very high molecular masses. It took chemists some time to realise that they were dealing with such large molecules. The German chemist Hermann Staudinger, working in the early 1900s, was the first to realise that compounds such as rubber were not just collections of tiny molecules held together by intermolecular forces, but were made up of huge molecules that contained many thousands of atoms covalently bonded together. It was thought at the time that a molecular mass of 5000 was the limit for any molecule, so it took some time for Staudinger’s model of a polymer to become accepted. In 1953, Hermann Staudinger (1881-1963) was awarded the Nobel Prize in Chemistry for his pioneering work with polymers.
The word polymer is derived from two Greek words: polys, which means many, and meros, which means a part. Polymers, then, are large molecules made up of many small molecules called monomers. The process of forming polymers from monomers is called polymerisation. Plastics –derived from the Greek word plastikos, which means shaped – is the general name for materials that, when heated under pressure, can be shaped or moulded or extruded (extruded means forced through a hole, for example to make rods or tubes). Not all polymer materials possess this property, so to avoid confusion the word ‘plastics’ is not used in this article until when I’ll be discussing about their environmental issues.
ETHENE AND ADDITION POLYMERISATION
Poly(ethene) – or polythene as it is popularly known – was discovered by accident in 1933. Two British chemists working at ICI, Eric Fawcett and Reginald Gibson, were trying to produce a ketone using benzaldehyde and ethene under very high pressure. They decided to leave the reaction at high pressure over a weekend, even adding more ethene when the reaction vessel sprang a leak.
Magmar452 • Public domain
By Monday morning, the hoped-for ketone had not been produced. Instead, there was a minute amount of a white waxy solid. They tried to repeat the experiment, but the reaction mixture exploded. The unpredictability of the reaction meant that making this unusual substance was very hazardous, so they stopped.
However, it was eventually realised that this was a polymer. Interest grew, and in 1935 Fawcett and Gibson made another attempt to produce it, this time with reactor vessels that could withstand very high pressures. By december, they had made 8 grams. They continued their experiments to find the optimum conditions. When they added cold ethene at a controlled rate, the reaction stopped going out of control. Soon they had enough solid polymer to establish that it could be melted and moulded.
Another British chemist, Michael Perrin, who took charge of the project in 1935, realised that oxygen was essential to produce this white waxy solid, polythene. (It is probable that oxygen had entered the original reaction vessel when it sprang its leak.) Perrin also showed that polythene was produced without the benzaldehyde of the 1933 experiment.
Polythene and the Second World War
The claim is often made that penicillin, the first antibiotic, was one of the chemicals that won the Second World War because it prevented terrible suffering and needless death from wound infections for thousands of soldiers. Polythene, too, had a valued role in the war. It was commercially produced in 1939, three years before penicillin. Unlike other insulators, it was impervious to rainwater and sea water, and one of its first uses was to insulate the electrical wiring of aircraft radar sets – radar being crucial to Britain’s survival in the war’s early years.
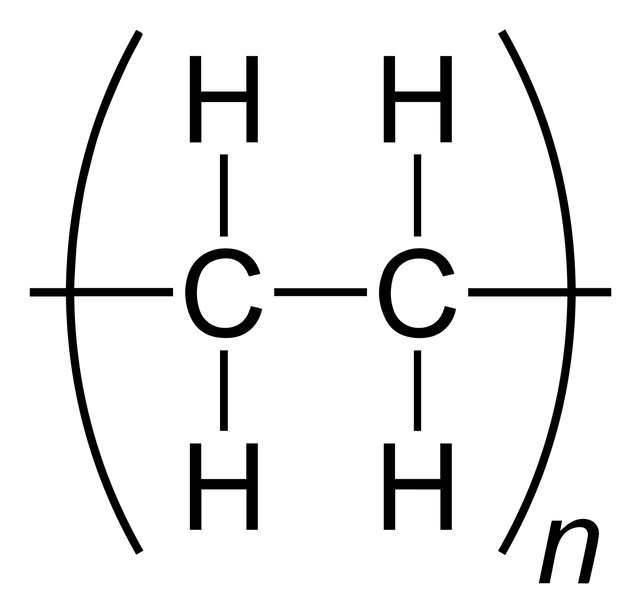
Poly(ethene) is called an addition polymer because, when the ethene monomers join together in the reaction, no small molecules are eliminated. The polymer is the only product.
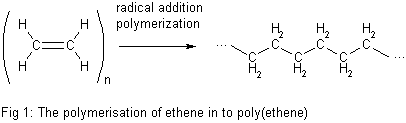
equation of polymerization of ethene into polyethene
Wikipedia, public domain
In the equation of the figure above, n represents a very large number. Poly(ethene) chains can be made up of thousands of ethene monomers, hence its systematic name. In the formula for the poly(ethene), the ethene unit is enclosed in brackets which cut the covalent bonds to show that we are representing the repeating unit of a very long chain.
TYPES OF POLY(ETHENE) AND POLY(PROPENE)
POLY(ETHENE)
Most people refer to poly(ethene) by its ICI brand name of polythene. It is the world’s leading polymer in terms of annual production, with more than 50 million tonnes of worldwide production and 650 thousand tonnes in the UK alone.
However there is more than one type of poly(ethene) that makes up this annual production.
LOW-DENSITY POLY(ETHENE)
The process invented at ICI to manufacture polythene produces branched chains. This means that the polymer chains do not all lie in parallel, as unbranched molecules could. Instead, they form a tangled mass. As the chains cannot lie close together, the density of this polythene is low. It is therefore called low-density poly(ethene) or LDPE.
The intermolecular forces that hold the poly(ethene) chains together are induced dipole –induced dipole forces. As the chains get longer, these weak forces act over a larger surface area. And as they become tangled, the tensile strength increases because when one chain is pulled it drags other chains along with it. However, because the branching keeps the chains further apart, LDPE has a lower tensile strength than polymers with no branched chains. Branching also keeps the melting point fairly low, at about 130°C. The process requires very high pressures of 2000 atmospheres, with a trace of oxygen or an organic peroxide.
Among the uses of LDPE are electrical insulation, tough transparent film in packaging, dustbin liners and carrier bags. However, the high pressure and a temperature of 200°C are very costly in terms of energy. A new process is producing a new form of poly(ethene) called LLDPE (linear low-density poly(ethene) with similar properties to LDPE but at a pressure as low as 1 atmosphere and a reduced temperature of 100°C. This makes the process greener (and safer too, as it avoids dangerously high pressures, with the potential to burst pipes in the plant).
HIGH-DENSITY POLY(ETHENE)
Linear chains of poly(ethene) – chains with no branching - can lie closer together than branched chains. This increases the density. High-density poly(ethene), HDPE was first produced in 1953 in Germany by Karl Ziegler while he was experimenting with an organometallic catalyst. This catalyst consisted of ethyl groups covalently bonded to aluminium atoms. Ziegler found that a little titanium(IV) chloride, TiCl4, mixed with triethylaluminium in an alkane solvent caused ethene to polymerise at room temperature and atmospheric pressure. Long linear chains of about 100 000 ethene units were formed. Today the conditions for HDPE production have changed a little.
Being linear, HDPE chains can form ordered structures by aligning themselves. This leads to large regions of crystallinity within HDPE, which give it a higher denity than LDPE.
The melting point and tensile strength of HDPE are also higher, and HDPE is harder than LDPE. The molecular regions that are not ordered are known as amorphous regions.
The uses of HDPE relate to its different properties. HDPE is more chemically inert and retains its shape at higher temperatures better than LDPE. This makes it ideal for containers for industrial and household chemicals and for milk. Almost half of the HDPE produced is blow-moulded to make bottles and other containers for a range of chemicals from shampoos to bleaches. Another quarter is injected into moulds (injection moulding) to make food storage containers, car petrol tanks, buckets and crates. It is also used for pipes that carry drinking water. Medical appliances, such as bedpans, are made from HDPE because its higher melting point allows high-temperature sterilisation without loss of shape.
LINEAR LOW-DENSITY POLY(ETHENE) (LLDPE)
The method of production of LLDPE is the same as for HDPE, using the same catalyst. This catalyst is now given the name Ziegler-Natta catalyst in honour of Ziegier and an Italian chemist, Giulo Natta, who used it to produce a particular type of another addition polymer, poly(propene). The polymer chains are linear, so to produce short branches to keep the chains apart and make a lower-density poly(ethene), a second alkene monomer is mixed with the ethene. This monomer is called a co-monomer and the polymer produced is a co-polymer. Co-polymerisation is often used to modify the properties of polymers. Two examples of alkene co-monomers are shown below:
• CH3–CH2–CH=CH2
But-1-ene
But-1-ene
• CH3-(CH2)3-CH=CH2
Hex-1-ene
Hex-1-ene
These co-monomers give short branches at intervals along the polymer chains, giving the polymer a low density because the chains cannot lie close to one another. Ziegler-Natta catalysts are of great economic and environmental significance, because they allow the use of much lower presures temperatures to produce polymers. For their contribution to polymer chemistry Ziegler and Natta jointly received the Nobel Prize for Chemistry in 1963.
POLY(PROPENE): ANOTHER ADDITION POLYMER
The equation for the addition polymerisation of propene is given in the figure below.
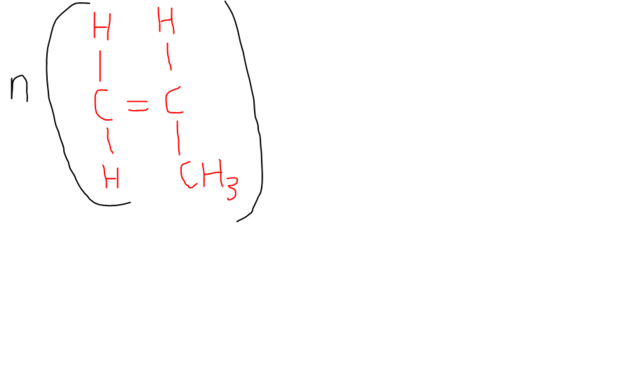
Wikimedia, Neutrinopenguin, C BY-SA 4.0
If all the methyl groups are oriented in the same direction, the polymer chains can get very close together and be crystalline. This form of poly(propene) is called isotactic to signify that all the methyl groups point in the same direction. It was produced by Giulio Natta using a Ziegler-Natta catalyst.
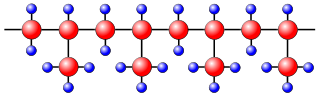
Wikimedia, Stan Zurek, public domain
In atactic poly(propene), which has no ordered orientation of its CH3 groups, the polymer chains cannot lie very close together. As would be expected, this form of poly(propene) has a lower melting point and is softer than the isotactic form. Isotactic poly(propene) is moulded into objects such as car bumpers and battery cases, and drawn into fibres for carpets and clothing. It is also made in sheet form for packaging. Isotactic poly(propene) is particularly useful for athletics wear because it does not absorb perspiration, but allows it to evaporate, unlike cotton clothing which absorbs and holds moisture. The atactic form is used in weather-proofing materials and sealants.
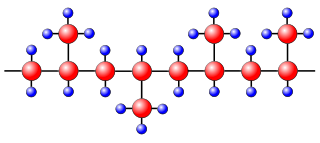
Wikimedia, Stan Zurek, public domain
PVC AND ADDITIVES
Other addition polymers whose monomers are based on the ethene structure can add together in a similar way. PVC or poly(chloroethene) is made from chloroethene monomers. The non-systematic name for chloroethene is vinyl chloride, hence the name by which the polymer was first known: polyvinyl chloride or PVC. When first made in 1912 in Germany. PVC was hard and brittle. These properties come from the chlorine atom.
Being highly electronegative, the chlorine atom forms a dipole with the carbon atom and the partial negative charge on the chlorine atom attracts a positive hydrogen atom, which also forms a dipole with the carbon atom. This gives rise to permanent dipole-permanent dipole forces which are stronger than induced dipole-induced dipole forces, making PVC stronger than poly(ethene).

Pixabay
The brittleness arises because the larger chlorine atoms tend to catch on each other when the polymer chains are pulled apart. Add to this that PVC often decomposed before it could be moulded, it is small wonder that the German company let its patent lapse in 1926. However, in the same year it was discovered that certain additives change the propertics of PVC, making it more flexible and easier to mould. As a result, PVC is now the world’s most versatile polymer and second only to Poly(ethene) in the amount produced.
The additives that make PVC more flexible and softer are called plasticisers. Plasticisers are molecules that get in between the chains, and enable them to slide over one another more easily. There is, however, concern that plasticisers in PVC food wrapping film may migrate into fatty foods and be harmful.
The PVC used in door and window frames is called uPVC where the ‘u’ stands for unplasticised, since flexible frames would not usually be a good idea. Another additive prevents the decomposition of PVC by ultraviolet light.
The use of additives to alter its properties means that uPVC is found everywhere. Credit cards are made from it, as are precision engineering items, flooring and footwear. If your house has polymer gutters, down-pipes and plumbing, the material is almost certainly PVC.
REFERENCES
polymer | Description, Examples, & Types | Britannica.com
Polymers in our daily life - NCBI
Polymer - American Chemical Society
PREVIEW 3:37 Monomers and Polymers YouTube
THE WORLD OF “POLYMER SCIENCE”
What is a Polymer? | MATSE 81: Materials In Today's World
From DNA to Silly Putty, the diverse world of polymers - Jan | TED-Ed
Thanks for writing this post. I didn't know anything about the history of the polymer discovery. In particular that they were made for the first time by accident (as many things in science). I am looking forward to read your next post :)
U're very welcome, Prof.
Actually, many of the great science discoveries were stumbled upon by accident, a term known as serendipity.
I've written a continuation on the WORLD OF POLYMERS, you can as well check it out. Here's the link; THE WORLD OF POLYMERS #2
Thanks for coming, @lemouth.
Thanks for the link to the second part. I had noticed earlier but didn't take the time to read it yet. I will read it maybe next week (am busy on other, offline, things at the moment :) ).
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Please consider setting @steemstem as a beneficiary to your post to get a stronger support.
Please consider using the steemstem.io app to get a stronger support.
Hi @empressteemah!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV