Chemistry and Metabolism of Biomolecules #8 : Metabolism of Glucose (Part I).
Introduction
 Honey: A Major Source of Sucrose (a disaccharide made up of fructose and glucose) (License: Public Domain]:
Pixabay
Honey: A Major Source of Sucrose (a disaccharide made up of fructose and glucose) (License: Public Domain]:
Pixabay Glucose. A very important molecule in intermediary metabolism. The versatility of glucose cannot be overemphasized.
Here’s a brief insight into how versatile glucose is. Glucose has multiple metabolic fates. For starters, glucose can be converted into glycogen or starch for storage for future utilization in supply of energy.
How exactly is energy gotten from glucose ? To answer that, I suppose a great number of us are aware of the biomolecule known as ATP (Adenosine Triphosphate). Maybe we are not aware that it is the energy currency of the cell but we have heard of it nonetheless.
Breakdown of ATP is an exothermic reaction (a reaction that leads to the release of energy either as light or heat) and thus, this process can provide the body with a whole lot of energy. The breakdown of glucose yields ATP. Connecting the dots now, it’s obvious how glucose can serve as a source of energy.
The breakdown of glucose also yields intermediates which can go into other pathways to yield other biomolecules. An example is seen where Dihydroxyacetone phosphate (DHAP), a product of glucose catabolism, goes into fatty acid synthetic pathways.
Another example is seen where Glyceraldehyde-3-phosphate goes into the thiamine synthetic pathway for the synthesis of thiamine (Vitamin B1).
Glucose can similarly be broken down through the pentose phosphate pathway, an alternative pathway to glycolysis (the main pathway for the breakdown of glucose), to yield the pentose phosphate Sugar, ribose-5-phosphate which is an important intermediate in the synthesis of nucleic acids (Your DNAs and RNAs).
I’ll like to highlight these various metabolic fates of glucose on the long run to show you how amazing biochemical processes really are while highlighting where they are connected in the process. To start the show; I’ll like to introduce you to;
The Glycolytic Pathway: The Main Reason Glucose Can Act as A Source of Energy
Have you ever been so weak physically that you had to take oral glucose solutions to get strong again ? Well, I’ve only had to do so after going jogging but the sudden restoration of energy after consuming oral glucose solutions will leave a curious mind wondering exactly how.
A carbohydrate rich meal is just a carbohydrate rich meal without various processes coming into play. When one takes a carbohydrate rich meal, a whole of processes occur before energy can be gotten from this meal.
First of all, amylases break down these carbohydrates which are mostly glycogen or starch to their respective monosaccharide unit, glucose in both cases. Glucose is absorbed into cells through special glucose transporters aided by the hormone insulin and it is now ready to be utilized. Pause. A special pathway known as Glycolysis or the Glycolytic pathway or Embden-Meyerhof pathway comes into play.
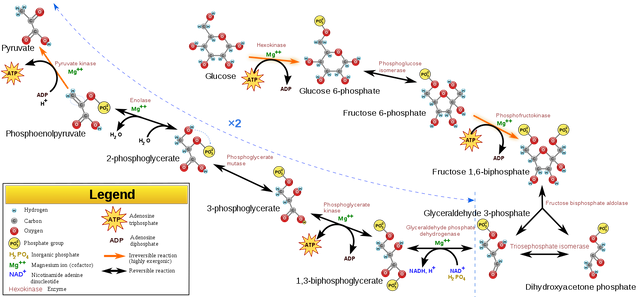 The Glycolytic Pathway showing the Preparatory and Pay Off Phases (License: CC-BY-SA 3.0, Author: YasmineMrabet]:
Wikicommons
The Glycolytic Pathway showing the Preparatory and Pay Off Phases (License: CC-BY-SA 3.0, Author: YasmineMrabet]:
Wikicommons Glycolysis is the pathway through which glucose is broken down in aim of yielding energy. In more specific terms, glycolysis is the breakdown of glucose to two molecules of pyruvate (a three-carbon sugar) which goes into various other pathways to yield energy.
The Glycolytic pathway is divided into two phases;
- The preparatory phase
- The pay off phase
The preparatory phase of glycolysis is the phase where ATP is being utilized instead of being produced. Red flag. Why is it a red flag ? The whole purpose of glycolysis is to produce energy through the formation of ATP which is an energy currency of the cell. Why are we now hearing of energy being used up in the form of ATP instead of the opposite ? No cause for alarm. As we progress you’ll see where it finally pays off.
The first step in the preparatory phase is the conversion of glucose to glucose-6-phosphate. Looking at the structure of glucose, an OH group is attached to its 6th carbon (C-6). An enzyme known as hexokinase can catalyze the movement of a phosphate group to this C-6 position to yield glucose-6-phosphate.
Where does this phosphate group come from ? you’ll ask. Well, from ATP (which is present in the cytosol where glycolysis takes place) which results in the formation of ADP and Pi. This is the where the first ATP molecule is used. This step is essentially irreversible. This is to ensure that once glucose goes into this pathway, it must be broken down to pyruvate, it’s ultimate product. Usually this is called the committed or rate-limiting step as it commits glucose, a molecule that can go into other pathways, to glycolysis
In the second step, a different enzyme comes into play. Phosphohexose isomerase. It catalyzes the conversion of the aldose glucose-6-phosphate to its ketose fructose-6-phosphate counterpart. This Step is very necessary as it leads to The positioning of an OH group at carbon 1, a requirement for formation of the next intermediate which involves the addition of another phosphate group.
The next step involves the addition of a phosphate group to fructose-6-phosphate. This is a rate limiting Step as it is essentially irreversible too. This is possible due to the catalytic action of the enzyme phosphofructokinase. ATP is similarly used up in this Step as there is a transfer of a phosphate group to the reacting molecule.
The product formed here is Fructose-1,6-bisphosphate. This molecule has two phosphate groups but so does Adenosine Diphosphate (ADP). Why is the former molecule described with the connotation bisphosphate while the latter with diphosphate ? In the former, the phosphate groups are attached to two different carbon atoms, carbons 1 and 6 while in the latter, the two phosphate groups are attached to the same carbon (carbon 5). Thus, the need for the different connotations to portray the common difference.
The fourth step in the preparatory phase of glycolysis involves an enzyme called aldolase. Here, the fructose-1,6-bisphosphate is split into two 3-carbon sugar molecules; Glyceraldehyde-3-phosphate (G-3-P) and Dihydroxyacetone Phosphate (DHAP).
Only G-3-P can continue in the Glycolytic pathway but it begs the question; what happens to DHAP ? Well, the cell is not wasteful when it comes to its resources so the fate of DHAP is seen in the last step of the of the preparatory phase.
An enzyme similar to phosphohexose isomerase seen in Step (2) above comes into play. G-3-P and DHAP are basically isomers so an isomerase enzyme can convert one to the other as deemed fit by the conditions of a cell. Triose phosphate isomerase catalyzes the isomerization of DHAP to G-3-P to continue in the Glycolytic pathway. At this point however, the pathway is split in two running side by side sharing identical reactions with formation of identical products ultimately leading to the production of two pyruvate molecules. Is the cell not amazing ?
The first step in the pay off phase involves the removal of a hydrogen molecule (also known as oxidation) from G-3-P with the addition of a phosphate group which comes from inorganic phosphate and not ATP in this case to yield 1.3 bisphosphoglycerate (phosphate groups at carbons 1 and 3). This step involves the formation of NADH since the removed hydrogen is donated to NAD+ while the other hydrogen atom is released into the cell as H+.
I mentioned earlier that a hydrogen molecule was removed from G-3-P. A hydrogen molecule is made up of two hydrogen atoms. Thus, the need for the donation of one hydrogen atom to NAD+ and the other into the cell.
The second step in the pay off phase involves the formation of ATP. Since this phase occurs as two separate but similar pathways due to the conversion of DHAP to G-3-P as explained earlier, two ATP molecules are formed technically. I know this may be too much to digest at this point. I’m sorry :). In this step however, there’s a transfer of the phosphate group from carbon 1 of 1,3-bisphosphoglycerate to ADP to yield 3-phosphoglycerate and ATP. The enzyme involved here is phosphoglycerate kinase.
In the next step, phosphoglycerate mutase comes in and moves the phosphate group on carbon 3 of 3-phosphoglycerate to carbon-2 yielding 2-phosphoglycerate. Mutases are the chief enzymes responsible for the movement of phosphate groups within a molecule. This is in contrast to kinases which are responsible for the movement of phosphate groups from one molecule to another.
In the fourth step, a water molecule is removed from 2-phosphoglycerate leading to the formation of phosphoenolpyruvate (PEP). Take note of this PEP molecule, it will come up a lot in the future. This is a dehydration reaction in technical sense since it involves the loss of water. The enzyme responsible for this reaction is enolase.
This Step is inhibited by fluoride. Flouride is a constituent of toothpastes so it’s no wonder now why we get hungry after brushing our teeth. The trick is, something is inhibiting glycolysis so Glucose cannot be utilized to supply energy.
Now, in the last step of the pay off phase and also the glycolytic pathway, pyruvate is formed. The phosphoryl group in PEP is transferred to ADP leading to the formation of pyruvate and ATP. In technical sense, two molecules of pyruvate as well as ATP are formed. I’m sure you can guess the enzyme that will be involved here. Yes, it just has to be a kinase. Pyruvate kinase in this case. Now pyruvate is formed. The question is; what happens to this pyruvate
Fate of Pyruvate in Aerobic and Anaerobic Conditions
What happens to pyruvate is hugely dependent on the presence or absence of oxygen (aerobic or anaerobic conditions respectively).
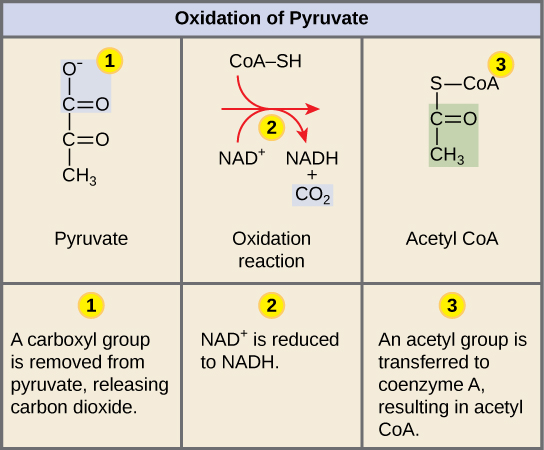 Conversion of pyruvate to acetyl coA (License: CC-BY 4.0, Author: CNX OpenStax]:
Wikicommons
Conversion of pyruvate to acetyl coA (License: CC-BY 4.0, Author: CNX OpenStax]:
Wikicommons In the presence of oxygen, pyruvate goes into the citric acid cycle via its conversion to acetyl-coA. A reaction catalyzed by Pyruvate dehydrogenase. This leads to the generation of more energy as the TCA cycle involves a series of energy generating steps.
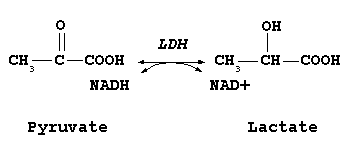 Lactic Acid Fermentation shoeing how lactate is produced from pyruvate (License: CC-BY-SA 3.0, Author: Jfdwolff]:
Wikicommons
Lactic Acid Fermentation shoeing how lactate is produced from pyruvate (License: CC-BY-SA 3.0, Author: Jfdwolff]:
Wikicommons 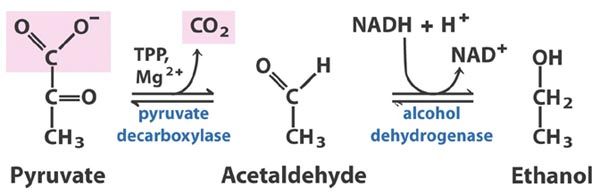 Alcohol fermentation showing the conversion of pyruvate to ethanol (License: CC-BY-SA 3.0, Author: Ronhjones]:
Wikicommons
Alcohol fermentation showing the conversion of pyruvate to ethanol (License: CC-BY-SA 3.0, Author: Ronhjones]:
Wikicommons In the absence of oxygen, pyruvate can either undergo lactic acid fermentation or alcohol fermentation. The former occurs in skeletal muscles while the latter in plants or microorganisms like yeast.
The formation of lactic acid from pyruvate in skeletal muscles is due to hypoxia (low oxygen supply). This happens mostly during exercise. Glycolysis occurs abundantly during exercise and thus there is need for NAD+ which can accept hydrogen atoms to ensure the blood doesn’t get too acidic. Under normal conditions, this NAD+ molecule is supplied in sufficient amounts and everything is fine. However during exercise, the supply of NAD+ is not enough as glycolysis is occurring too many times and this NAD+ has to be gotten through extreme measures. With the low supply of oxygen observed during exercise, pyruvate is converted to lactate, a reaction catalyzed by lactate dehydrogenase which involves the addition of hydrogen to pyruvate to form lactate. NADH supplies this hydrogen and thus it is converted to NAD+.
This NAD+ feeds into the Glycolytic pathway immediately keeping the NAD+ supply constant. Speaking of efficiency at its best.
In plants or microorganisms however, the pyruvate is converted to ethanol. This is possible because these organisms contain an enzyme known as pyruvate decarboxylase which converts pyruvate to acetaldehyde releasing CO2. The released CO2 is the reason why carbonated drinks and your favorite champagne drink fizzles when opened. Organisms which do not contain the pyruvate decarboxylase enzyme cannot carry out alcohol fermentation.
The acetaldehyde is acted upon by alcohol dehydrogenase leading to the formation of ethanol, an alcohol. Well, thanks to brewer’s yeasts our parties don’t have to be boring.
Summary
Glycolysis is a very important pathway in intermediary metabolism. Glucose has been shown to go through various routes of metabolism for the purpose of supplying energy. Did you find this enlightening ? Thanks for coming around again. I look forward to the next installment of this series with all of you.
References
Lehninger, A. L., Nelson, D. L., & Cox, M. M. (2000). Lehninger principles of biochemistry. New York: Worth Publishers. pp. 521-534.
Chatterjea, M. N. (2012). Textbook of Medical Biochemistry. (8th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 327-330.
Vasudevan et al. (2011). Textbook of Biochemistry. (6th Edition). Jaypee Brothers Medical Publishers. New Delhi. India. pp. 92-102.
Image Sources
All images are from pixabay and wikicommons licensed under creative commons and eligible for commercial use.
I'm a proud member of the steemstem community which promotes quality posts in the science, technology, engineering and mathematics fields on the steem blockchain mainly through interaction and engagement. Feel free to join us on discord here
Glucose is a great carbohydrates because it provide us energy like what stated in your article. Some patients with low energy use glucose into their blood in order to provide energy for their bodies. But always remember, too much glucose may cause hyperglycemia that is a symptom of diabetes.
For those who became amazed of glucose, please be aware of the amount you eat.
By the way, @kingabesh, I learned new from you post! Since I began in my elementary days, I didn't know how glucose can provide us energy but thanks for this. I knew now how energy is stored in the bonds of glucose.
Resteemed, followed, and upvoted! ^_^