On the trail of Heat (II)-- Understanding Heat transfer and the modes of propagation.
In the previous article, we discussed the basic concept of energy, and how it affects life. We drew a conclusion that energy is indeed nature's money, and that there wouldn't be any work done without it. We also discussed an important form of energy: Heat (also referred to as thermal energy), as well as temperature and the temperature scales. Basically, what happens when heat is imparted into an element is that its temperature increases.
Today, in the spirit of science, I'll be explaining how heat energy travels or is transferred from one point to another, and how science tracks or measures this transfer. This branch of science is known as heat transfer which by definition is;
The study of the generation, utility, motion, and conversion of heat between Physical systems -Wiki.
Now, even though we cannot see heat move, a simple experiment of placing one's hands on a pan over the fire would make quite the ideal impression. Hopefully it wouldn't be a lasting one. would depend, just don't hold on for too long.
Another way one could attest to the transfer of heat is the very essence of sitting by the fireplace on a cold rainy night. Though not in contact with the flames, You get all warmed up and cozy. We'll be giving life and scientific terms to these explanations as we move on.
Now, here's the thing about thermal energy transfer: At the macroscopic level, it only happens whenever there is a difference in temperature between 2 material bodies, and it occurs in one direction by default. That is, from a hotter region to a colder one, or from a hot body to a relatively colder one. Which would explain why you feel warmer when close to the fireplace than not. If the heat source in non-renewable, thermal energy is transferred between matter until all the bodies concerned reach equilibrium, which conforms to the Zeroeth law of thermodynamics that states that:
If two bodies are each in thermal equilibrium with some third body, then they are also in equilibrium with each other. - Source: Livescience.
So two bodies involved in heat transfer would eventually settle in thermal equilibrium (at the same temperature).
Hence, at the macroscopic level, we see temperature change as heat is transferred. But how about we consider heat transfer at the microscopic scale? what do we expect?
We're probably aware of the fact that every molecule above absolute zero is always in a constant state of motion. If this isn't familiar to you, I stated this in my previous post I titled What's hot or not.. You may as well check it out. At the microscopic level, the molecules in solids vibrate about their mean positions because they're bound together in a lattice. Whereas, the molecules in liquids and gases are freer to move about. As thermal energy is imparted at one point, these molecules increase in speed and tend to move about more frantically. The energy gained is transferred from one particle to the next, and soon, the other molecules are excited as well and vibrate faster. Thus, heat energy is transferred.
However, as we shall soon discover, the above explanation cannot hold a candle to the complete description of how thermal energy flows. And that is because thermal energy sometimes doesn't even require vibrating or moving particles for propagation. Hence, it can travel through space in an electromagnetic manner. Which is how the heat from the sun gets to us in the first place. this is known as radiation, and we shall consider it later in this article.

Modes of heat transfer.
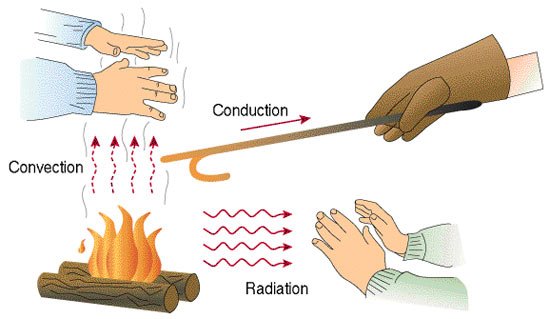
Science groups heat transfer into 3 categories which is referred to as the modes or conditions of heat transfer. Basically, heat transfer is:
- By Conduction.
- By Convection.
- By Radiation.
Conduction.
Conduction is the transfer of thermal energy through a body, or between bodies of different temperature in contact. By holding on to a hot cup of coffee in a ceramic container on a cold day, you would discover that your hands get warmer as the seconds pass. This is simply because the average cup of coffee is much higher in temperature than the average human body temperature. As the fast-moving molecules of the coffee are in constant contact with the ceramic container, its particles get hotter and transfer that energy to the molecules of your palms. Hence, your sensory organs interpret this change as a rise in temperature.
The operative word to note here is contact. because it is the one necessary condition for heat transfer by conduction, as propagation involves the direct interaction between particles of one solid to the next.
While some materials are good at allowing heat flow through them in varying degrees, others aren't at all, and this is due to the thermal conductivities of these materials. Thermal conductivity is the term that tells us which material is relatively better at allowing the flow of heat through them than the others. Hence, these materials that conduct heat are known as Conductors, and the ones that do not conduct heat are known as insulators. These terms apply in the electrical field as well.
Metals such as Aluminium are better conductors of thermal energy than materials such as wood and glass, which is why they take longer to heat up and to cool off.

Convection.
Convection is pretty much the same idea as conduction, the only notable difference is that it relates to fluids alone. As fluids are heated, they lose viscosity and density and move faster and further apart, carrying along the heat energy with them.
Thermal energy flows through a fluid in a pattern we call convection current, which is basically the rise of less dense fluid to the surface level, and the sinking of denser fluid particles to the bottom. Cold fluids are generally denser than warm fluids and as their temperature approaches absolute zero, they solidify. For example, ice. However, by applying heat to fluids, as in a kettle of water, their particles increase in speed and expand to take up more volume and space. With time, the convective current is set-up and the warmer fluid rises to the top where it gives off a bit of that energy as losses to the surrounding. Simultaneously, the fluid particles at the bottom of the kettle closest to the heat source is heated and rises to the surface to replace the previous one, Thus creating a rolling convective current, which is really an important aspect of boiling, evaporation, land and sea breezes, and the earth's geomagnetic field deep down in the outer core.
So, while conduction is possible by the contact between vibrating particles of solid matter, convection is possible by the full Brownian motion of the particles of fluid matter.

Radiation.
The propagation of thermal energy doesn't always require matter for propagation as I stated earlier, and this is the very basis of heat transfer by radiation. Unlike Conduction and convection that require a medium, Radiation is the transfer of heat energy by electromagnetism as a member of the spectrum known as Infrared waves. Radiation occurs for any frequency. Not only the infrared ones. And all radiation yields heat transfer (but not all with the same efficiency of course).
Again, the aim of every heat transfer process is by the stimulation of molecules to vibration or movement, and radiation is no different. Even though it requires no medium for propagation, as infrared waves strike a body, it makes the particles of that body vibrate faster thus making it hot. This heat can then be further propagated by conduction or convection, depending on the medium in question. Hence, it is safe to say that radiant heat is the first mode of heat transfer since energy comes from the sun at over 148 million Km from the earth.
It should be noted that radiant energy is a lot different and shouldn't be confused with radioactivity at all. The earlier is thermal energy from the sun which is absorbed and reflected by the earth's surface, while the latter is a lot harmful to life and is given off by radioactive elements such as uranium and radon.
Every hot body gives off radiant energy when they are hot, regardless of whether they are isolated in a vacuum or not. This phenomenon has been applied in early Infrared data transfer technology in computers, and in the photoelectric effect.

How does science measure heat and heat transfer between bodies?
These methods were created in order to answer the questions that relate to the thermal conductivities of materials. For example, Why exactly are copper rods better heat conductors than wooden ones even though they have the same mass? why is the movement of heat in some materials faster than others?
Basically, we have two concepts that seek to explain why this is possible, they are:
- The specific heat capacity.
- The calorific value (Calories).
Specific Heat Capacity (S.H.C).
Due to the nature of some materials (either solids or fluids), they would require different amounts of energy to increase their temperature to a particular reference or fixed point. Thus, one excellent explanation for the heat conductivity of a material is the S.H.C, which is basically defined as: The amount of heat required to raise the temperature of a unit amount of a material by 1oC.
I highlighted the keywords in that definition: "Unit" stands to indicate that the specific heat capacity pays attention to a fixed amount of every material, which is usually taken as 1g. The S.H.C. shouldn't be confused with the Heat capacity of a material, which is the amount of heat required to raise an overall body in complete equilibrium to the temperature of 1oC.

The Calorific value.
Another interesting way that heat transfer is measured is by the calorie, which is also regarded as another unit of thermal energy just like the joule.
The calorie is equal to 4.186 Joules and just like the S.H.C, it is the amount of heat required to raise the temperature of a 1g of a substance by 1oC.
Hence, if you're not working with the SHC, you could work with the calorie. One unique use of the calorie is in a procedure in chemistry called Calorimetry, which simply is a way of determining the amount of heat that is given off of a burnt substance. the instrument in which this procedure would be carried out is called a calorimeter.
In modern times, the calorie has mostly gained adoption as a means of measuring energy content in edibles and has found application in Food processing, health and fitness, etc. It is written all over packs of foods and energy drinks.
It should be noted that there are quite similar terms that one needs be careful of here. That's in the use of the words:
- calorie (note the small lettered c.) in grams.
- Calories. in kilograms
The calorie is the basic unit of energy which is equivalent to 4.186 J as described earlier. It is measured as 1 gram. Whereas, the Calorie (with an uppercase C) is simply the calorie value in Kilograms. This is because the calorie has very negligible values, so food scientists prefer a more significant number to make for less cumbersome data and calculations.
1000 calories = 1kcal = 1Cal

Conclusion:
In all, energy can only be useful when it is being transferred or put to work. Most times, work includes one of the three modes of energy transfer. - Either by conduction (which involves contact), by convection or by radiation-. An unlit matchstick has thermal energy stored away and would be totally useless until it is struck against a lighter. Then it gives off that stored energy by combustion (burning) and radiation.
Heat transfer is highly important to life. Evaporation of seawater from the surface of the ocean at every point in time, which of course, falls back as rain in regions that aren't close to oceans help sustain life and reduce desertification. Ocean currents, an important phenomenon of convection in the sea helps raise nutrients from deep down the ocean floor to corals to enable sea life flourish. By studying heat transfer processes, humans have been able to come up with even simpler, smarter and better materials and technologies. Think Silicon and ICs for computation. Think microwave ovens, boilers, refrigerators and smart window panes for homes and offices. Think Fire-resistant paints, textiles, better tyres for construction clothing and transportation respectively. Need I go on?.
With this, I think we've got the basic idea about how the propagation of thermal energy works. Regardless of which form of energy transfer you get to interact with every day, each mode is of equal importance to life on earth. Consider this a more simplified approach to the concept of heat which you were introduced to sometime back in high school. Welcome to the blockchain! Stay close.
PS: I think this animated video could help simplify things if you aren't so much in the reading mood at the moment. Yeah, it's for 5th graders.
Thank you for reading.
References:
1. Wiki -- Zeroeth law of thermodynamics.
2. Wiki -- Heat transfer.
3. Youtube -- Heat energy transfer.
4. 800MC --The Joule, the calorie and the SHC.






Missing my P. N OKEKE
Physics is actually quite interesting, just wasn't so good then..
Learning more from you and @greenrun now.
I guess I don't need my textbook, I have got the brainiacs on physics on steemit
Those books were pretty much useless at explaining the concept itself than the math. They really focused on the math, which shouldn't really be the case.
Consider this P. N. Okeke on steroids.
Thanks for coming around.
All those oyinbo textbooks are better
Hi @pangoli!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Just one remark:
Radiation occurs for any frequency. Not only the infrared ones. And all radiation yields heat transfer (but not all with the same efficiency of course).
Thanks for pointing that one out sir. I have included it in the article. :-D
No problemo my friendo :)
Nice one,
Anyone up to answer my question? :-D
Interesting read. This brings to memory the days of high school physics
To answer your question, most times, the scales can be used interchangeably but I guess Celsius is used in this case because the very grassroots(absolute zero) in kelvin scale is just an ideal thing and not actually possible in real life situation. Celsius however offers negative values up to - 273.