CELL SUSTENANCE #3: globulin and immunoglobulins; the Guardian polypeptides.
Hi everyone, very nice week ahead of us and welcome to the third part of this series on #steemstem community. Today we will discuss about the proteins which guard our body, little knowledge of each of them will be essential for adequate understanding of other parts of this series ...
After the precipitation of proteins in the laboratory using salts, these separated proteins are analyzed by electrophoresis. Electrophoresis involves the movement of charged particles through an electrolyte when subjected to electricity; albumins are negatively charged and thus are mobilized towards the anode, cellulose acetate is used as a supporting medium to permit resolution. After staining, these proteins are separated into five bands.
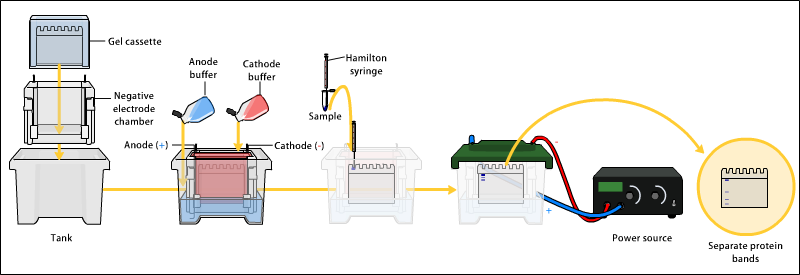
A gel electrophoresis. Image source: ">a href=". CC3.0 share-alike license. Contributed by Bensaccount
Namely
The Globulins are observed to be bigger in size than Albumins which have already been described, Globulins constitutes all the fractions observed in the electrophoresis of precipitated proteins except the Albumins. The various fractions of globulins differ in their various actions. They include:
The α-globulins:
The α1-protease inhibitor Also known as α1-antitrypsin; the protease inhibitor is the major component of the α1-globulins and accounts for over 85% of this fraction of globulin plasma proteins. It inhibits protease such as chymotrypsin, elastase and trypsin, this action protects the body tissues from being destroyed by these proteases
The α1-fetoprotein which is the component of α1-globulins are normally found in the fetal serum and tissues where they play an immunoregulatory role. Presence of the fetoprotein in am adult serum is an indication of neoplasia in the liver.
))
A retinol binding protein credit: wikimedia Creative commons license. Contributed by Fvasconcellos
The Retinol binding protein; Vitamin A are obtained from the diet, they are known as Retinoids due to their role in normal eye site. Through redox reaction, retinol is synthesized from retinal. After this synthesis, there is the need to transport this retinal to the area where it performs it’s action –the retina. Retinal is bound to retinal binding protein which serves as the carrier for this transport.
The High density lipoprotein which helps in the esterification of cholesterol, it transports cholesterol from the extrahepatic tissues to the liver.
The α2-globulins:
The Haptoglobin binds to haemoglobin to form the haptoglobin-hemoglobin complex, this complex is too large and can not be filtered by the kidney as its size exceeds the range of size of particles which can pass through the renal glomerular membrane, hence the kidney is unable to remove haemoglobin from circulation, this prevents the loss of iron via urine, hence iron is conserved. It has a half life of fifteen (15) days.
))
The thyroid gland credit: wikimedia Creative commons license. Contributed by Arnavaz
The thyroid gland is a special organ located in the neck region from the C5-T1 vertebrae; this organ is tasked with the production of thyroid hormones. Hormones are endocrine substances thus their area of utilization is usually far from the area of production, there is the need for these hormones to be transported to the target organ; the execution of this task is aided by the Thyroxine binding globulin.
The Macroglobulin very much like the α1-protease inhibitor, the macroglobulin also inhibits protease such as collagenases, trypsin and chymotrypsin; this also has the same function as the α1-proteases inhibitor–protection of the tissues from being digested by proteases.
The Ceruloplasmin has an oxidase activity, with the ability of oxidizing iron from ferrous to ferric oxidation states, it also regulates this action and hence is essential in regulating the utilization of iron, ceruloplasmin also transports copper.
The β-globulins
Haemopexin has a similar function and mode of action as the haptoglobins, hence it prevents the loss of iron in urine.
Transferrin is a transport protein synthesized in the liver which binds and transports two (2) molecules of ferric iron to the site where they are needed, usually for the synthesis of the harm part of haemoglobin.
))
The microglobulin. credit: wikimedia CC3.0 license. Contributed by Atropos235
C-reactive proteins: C-reactive proteins are positive acute phase response proteins, it is released only in response to bacterial infections, this is because it forms precipitate with somatic C-polysaccharide of pneumococcus bacteria.
The Microglobulin are important part of the Human Leukocyte Antigen (HLA), the human leukocyte antigen is the human form of the Major Histocompatibility Complex (MHC) hence it plays an important role in the immune cells’ recognition of antigens.
The Gamma Globulins :
The gamma globulins are mainly immunoglobulins and the C-reactive proteins, the C-reactive proteins shuttles between the beta and gamma fractions during electrophoresis and can be found in any or both of these two fraction, that have already been discussed with the β-globulins.
Immunoglobulins on the other hand are popularly referred to as antibodies, however all antibodies are immunoglobulins but not all immunoglobulins are antibodies, but they are chemically related to antibodies. Antibodies are glycoproteins produced by B-lymphocytes in response to stimulation by immunogens. Immunogens are those high molecular weight substances which can bind to antibodies and initiate an immune reaction.
Hence for a shorter description , antibodies are immunoglobulins formed in response to immunogens. Antibodies are present on the membrane of B-cells and they bind to antigen/immunogens and directs the synthesis of antibodies similar to itself, hence antibodies identical to the one that binds to the immunogens are produced by the B-lymphoid cells. Immunoglobulins consists about 20% of the total plasma proteins and are very important part of the adaptive
and innate immune system.
STRUCTURE OF IMMUNOGLOBULINS
All immunoglobulins have the same basic structure , the basic monomeric structure of the immunoglobulins consists of two light chains and two heavy chains of amino acids. The light chains are identical to each other, the heavy chains are also identical to each other.
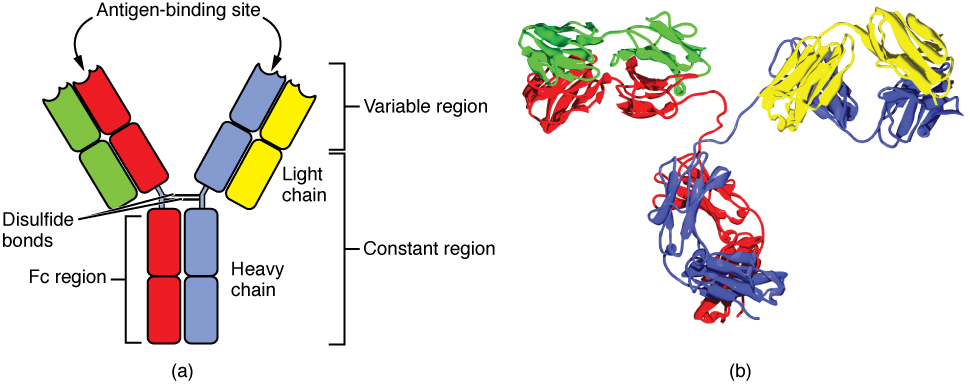
heavy and light chains of immunoglobulin. Image source wikimedia. CC3.0 license. Contributed by OpenStax College.
Immunoglobulin light chains are polypeptides of about 25KDa molecular weight, they consists of a constant region and a variable region ; the constant region has a relatively constant amino acid configuration /sequence, however, the amino acid sequence in the variable regions varies greatly.
The light chains are classified into Kappa and Lambda light chains depending on the amino acid sequence of the constant region, an antibody contains only one type of light chain and never both of them, 60% of antibodies in the body are of the Kappa light chain ; Lambda light chain accounts for the remaining 40% of light chains in the total antibodies of an individual’s immune system ; the lambda light chain is of six subtypes denoted by numerals 1-6, but in essence, there are no difference between the kappa and lambda light chains and even the six subtypes of the lambda light chains in terms of function.
The immunoglobulin heavy chains are large polypeptides with molecular weight of about 50KDa,the heavy chains distinguishes the immunoglobulins into different subtypes ; it consists of one variable region and about 3-4 constant regions, these regions are also known as domains. Variations in the amino acid sequence of the constant regions of the immunoglobulins heavy chains forms the basis of their division into five subtypes, these five subtypes differ in physical and biological properties and unlike the light chain, they perform different functions, this enables the immunoglobulins to function in different types of immune response and at different phases of the response too. The differentiates immunoglobulins into:
These subtypes differs in type and also amount of amino acids it contains.
The light chains and heavy chains are held together by disulphide bonds, hydrophobic bonds, salt linkages and hydrogen bonds ; these chains are held in position by the bonds to form a ‘Y’ configuration of the immunoglobulins. The constant regions are in the carboxy terminals while the variable regions are arranged towards the amino terminal if the Immunoglobulin.
In laboratory practice, the immunoglobulins can be digested by certain enzymes such as pepsin and papain. Pepsin cleaves the immunoglobulins toward the carboxy terminal wHile papain cleaves the immunoglobulins toward the amino terminal.
Cleavage by papain enable the study of immunoglobulins in parts as it cleaves it into parts of two identical fragments, these two identical fragments has the ability has the ability if binding antigens and hence are termed the Antigen Binding Site (fragment of antigen binding), these parts binds to the antigen, they contain a constant region, a variable region and the amino terminal, the variable regions are the point where the antigens binds to, they confers specificity to the immunoglobulins.
))
Polymers of immunoglobulin credit: wikimedia CC2.5 attribution-share alike license. Contributed by Martin Brändli
The third fragment is observed to crystallize when frozen, hence the term ‘fragment crystallizable’ it contains the remaining constant regions and the carboy terminal and performs most biological activities of the immunoglobulins, it relays information about the bound antigen to the test of the immmune system.
Immunoglobulins in the body fluids can also be polymeric. An Immunoglobulin polymer is formed by the union of two or more basic immunoglobulin units. This union is made possible by an additional polypeptide chain known as the J-chain, the J-chain is an elongated glycoprotein of approximately 15KDa molecular weight ; it has a high quantity of aspartic and glutamic acid residues. The immunoglobulins in polymerization has the fragment crystallizable region (FC région) arranger toward the centre with the fragment of antigen binding arranged towards the periphery, this feature can be seen in polymeric immunoglobulin A and Immunoglobulin M.
REFERENCES
- What is immune globulin? -bdipharma
- Globulin -Wikipedia
- Alpha globulin -sciencedirect
- Gamma globulin -wikipedia
- Immunoglobulin Structure and Classes -thermofisher
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on steemit chat or discord here. If you are from Nigeria, you may want to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem. Please also check this blog post from @steemstem on proper use of images devoid of copyright issues here.
