Answer: What are some statistics that show increased risks after getting the covid vaccines? (Part 3)
Sub-clinical Myocardial Injury
One scarcely researched and rarely discussed AESI is sub-clinical injury to the muscular tissue of the heart of recipients. Perhaps because it appears as either mild to asymptomatic cases that are only detected through electrocardiogram and PET scans this AESI is generally ignored despite being very common in recipients based on the available literature occurring in a few percent or more of booster recipients.
A prospective active surveillance study of University Hospital Basel healthcare workers (n = 777) myocarditis-related symptoms, using venous blood samples, after receiving the first booster was matched with 3,716 unboosted control group participants from December 2021 to February 2022. The study found that 2 of the 777 hospital workers, both female, suffered vaccine induced myocardial injury and chest pain that met the Brighton Collaboration case definition Level 2 myocarditis (1 case in 388.5 recipients), while 22 hospital workers overall were found to have vaccine induced myocardial injury with 91% of cases occurring in women with a median age of 46 years (1 case in 35 recipients). 40 boosted hospital workers had cardiac troponin levels higher than their sex specific normal upper limit (1 case in 19 recipients). All 777 boosted healthcare workers had statistically significant higher cardiac troponin concentrations compared to the unboosted control group three days post administration.
Another prospective active surveillance study, published in European Journal of Heart Failure, of the myocarditis-related symptoms of Israeli hospital workers who received the second booster (n = 324) found, through collected blood samples analyzed for cardiac troponin levels, found 134 adverse events including 7 heart palpitations and 2 participants who had post administration cardiac troponin levels more than 50% higher than their baseline measurement indicating vaccine induced myocardial injury (1 case in 162 recipients). One participant had symptomatic myocardial injury including fever, chest pain and muscle soreness while the other participant was asymptomatic.
A Thai study, published in Tropical Medicine and Infectious Disease assessed post jab ECGs and self-reported cardiac symptoms after each of the two doses of ModRNA in 301 adolescent participants of both sexes between 13–18 years of age with a mean age of 15 years. After the second dose, 3 participants were diagnosed with myo/pericarditis and pericarditis, 2 participants were hospitalized (one for 2 days and one for 7 days) and 1 ended up in the ICU. In total the study found one incidence of myopericarditis (1 in 301), 4 incidences of subclinical myocarditis (1 in 75), and 2 incidences of pericarditis (1 in 151).
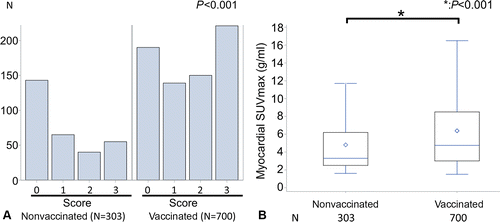
A retrospective PET Scan study (n = 1003) of both vaccinated (n = 700) and unvaccinated (n = 303) asymptomatic participants, published in Radiology, found that vaccinated participants had higher fluorine 18 fluorodeoxyglucose uptake in the myocardium (muscular tissue of heart) than unvaccinated even after adjusting for age and cancer diagnosis status up to 180 days after the second dose. Within the vaccinated group the myocardial visual score was higher in patients that reported a sore arm after injection than those who did not and for 16 patients with PET scans conducted before vaccination the myocardial visual score was confirmed to be higher post vaccination.
Even though the Swiss Hospital Study found that boosted hospital workers developed myocardial injury post booster at an alarming rate of nearly 3% of recipients (22 out of 777) the authors claimed:
Before the COVID-19 vaccine was available, the incidence and extent of myocardial injury associated with COVID-19 infection was much higher than observed in this active surveillance study after booster vaccination.
However, as Klement and Walach note in a recent reanalysis published in The Egyptian Heart Journal, this can only be asserted of persons ever HOSPITALIZED with COVID19 not anyone who has had a SARS-COV-2 infection because all three studies cited in support of that statement consider only hospitalized COVID19 patients which excludes the vast majority of the general public especially younger age groups who are more likely to be infected than their elders but far less likely to even develop symptoms or progress to severe disease requiring hospitalization. The three studies were also published prior to omicron and thus limited their purview to pre-omicron variants with the latest one, also published in the same European Heart Journal, being revised as a final draft at the beginning of the Delta variant dominance. Their study parameters not only make their findings inadequate to generalize to the entire population of infected persons the time they were conducted also makes them extremely anachronistic given how rapidly the virus has mutated from the ancestral variant and other pre-omicron variants.
A prospective registry based observational study (n = 200) conducted by the Polish Cardiac Society (SILCOV-19), found that only 1.8% (2 out of 114) of non-hospitalized COVID19 patients had elevated Troponin T levels compared to 5.1% (40 out of 777) of boosted healthcare workers in the Swiss Hospital Study and 7% of hospitalized COVID19 patients in the same SILCOV-19 study. The SILCOV-19 study found only 2 cases of myocarditis both in hospitalized patients with no clear indication that infection was the cause. Keep in mind the SILCOV-19 study data are from June 2020 to March 2021 when the ancestral wild-type variant through beta variant, which are several fold more virulent than any omicron sub-type, were circulating. Thus, the rate of elevated troponin T levels and myocardial injury from omicron infection would be even lower today.
A much larger prospective multicenter cohort study (n = 19,378), published in the Journal of The American Heart Association, that used the Outcomes Registry for Cardiac Conditions in Athletes that aggregated data on student athletes infected by SARS-COV-2 across 42 universities, found that the majority of infected student athletes were either asymptomatic (33%) or had very mild symptoms (29%). Of the 3,018 student athletes who underwent any cardiac screening after infection only 137 (4.5%) had any abnormal testing while only 81 (2.7%) had abnormal cardiac testing possibly related to infection, 14 (0.46%) had cardiac magnetic resonance abnormalities possibly related to COVID19 and only 2 (0.06%) had multiple abnormal tests. The study authors identified definite, probable or possible SARS cardiac involvement in 21 of the 3018 student athletes (0.7%) tested and estimated the prevalence of cardiac involvement in student athletes infected with SARS-COV-2 to be around 0.5% to no more than 3%.
I brought up the prior study of infected student athletes because although they don’t represent the general population of SARS-2 infected persons they are one of the few remaining segments of the population that is financially coerced into getting boosters. As of the fall of 2023 almost 100 colleges mandated bivalent boosters while 70 of the top 800 U.S. colleges will require the new XBB1.5 booster, against a predominant JN1 variant that is 35 mutations different just on the spike protein, in the Spring of 2024. This is one of the reasons why discourse about modRNA products remains salient despite federal courts striking down mandates for the vast majority of the general public and most health ministries in the rest of the world scaling back booster recommendation to immunocompromised individuals and senior citizens.
In December 2022, I covered one of the first benefit risk assessments of booster mandates for college students, published in the British Medical Journal, in my post When The Treatment is Worse Than The Disease. Based on available literature on hospitalizations and SAEs following both the primary series and boosters for adults between 18-29 years of age, including multiple national cohort studies and an update to the CDC’s data for the prior delta variant, the authors estimated a number needed to vaccinated (NNV) to prevent 1 hospitalization in this age cohort to range from 31,207 to 42,836 over a 6 month period. For that many recipients, there would be 18.5 SAEs or 593.5 SAEs per million doses compared to 32 hospitalizations averted in this cohort per million doses based on the SAEs attributable to the shot in Pfizer’s original clinical trial. With 28.3% of ModRNA recipients self-reporting being unable to carry out daily activities following the booster dose, we could expect 45,752 to 107,784 reactogenicity cases per million booster doses and 47.6 to 147 cases of myocarditis or pericarditis per million booster doses for 23-32 hospitalizations averted by booster per million doses in this age cohort.
The Kaiser Permanente Cohort Study of adolescent members who received a booster at least 5 months after the primary series (n = 65,785) identified 6 recipients (1 case per 10,964 recipients) who met the CDC case definitions for acute myocarditis or pericarditis within 21 days of their booster shot, all Pfizer products, with 4 cases among 27,253 male recipients (1 case per 6,813.25 recipients) and only 1 “mild” case. Based on this data the study authors estimate a risk of 9.1 cases of myo/pericarditis per 100,000 booster doses for all adolescents and 14.7 cases per 100,000 booster doses for male adolescents or 91-147 cases per million booster doses.
A nationwide cohort study of adolescents and adults between 12-39 years beginning in December 2020 in four Nordic countries (n = 8.8 million), published in the European Heart Journal, identified 1,533 cases of clinical myocarditis (82% of cases were identified in males) corresponding to an incident rate of 1 case per 8,000 person years of follow up or 1 case per 5,779 recipients with a median hospitalization length of 3 days for myocarditis following Pfizer primary series and booster and 5 days for myocarditis following Moderna primary series and booster over the two year study period. Compared to the unvaccinated cohort (n = 1.6 million), the incident rate ratio of myocarditis for males following all 3 Moderna doses was 6.47 and 12.56 for the Moderna primary series alone. The same incident rate ratio of myocarditis for males following all 3 Pfizer doses was 2.21 and 2.86 following the primary series alone. For females the relative risk was much lower with incident rate ratios of 2.56 following the Pfizer primary series and 3.14 following the primary series and booster compared to unvaccinated females.
A pre-omicron nationwide cohort study of adolescent primary series recipients in Hong Kong (n = 343, 700), published in the Journal of the American Medical Association, found that myocarditis occurred at an incident rate of 3.12 cases per 100,000 recipients for the first dose and 22.15 cases per 100,000 recipients for the second dose with 84% of hospitalizations occurring after the second dose. After implementing a single dose policy for adolescents, Hong Kong decreased the rate of shot induced myocarditis from 1 case per 4,705 recipients to zero new cases. There were no deaths with COVID19 and only one hospitalization requiring admission to a pediatric intensive care unit among the adolescent cohort in this study, during the course of the pandemic, while there were 43 adolescent hospitalizations following the first and second dose of the Pfizer primary series.
I have covered prior nationwide cohort studies of shot induced myocarditis in (Part 1) (Part 2) (Part 3) (Part 4) and (Part 5). As I mentioned in (Part 6), an Israeli study conducted among patients of Clalit Health Services (n = 787,968) that compared background rates of myocarditis and pericarditis within the general population, using a test negative control, to those of patients with PCR or antigen test confirmed SARS-COV-2 infections between March 2020 and January 2021 found no statistical difference in the incidence rate of both myocarditis (p =1) and pericarditis (p =0.17) was between the COVID-19 cohort and the control cohort (at an alpha level of 0.05).Evidence for Toxic Lots
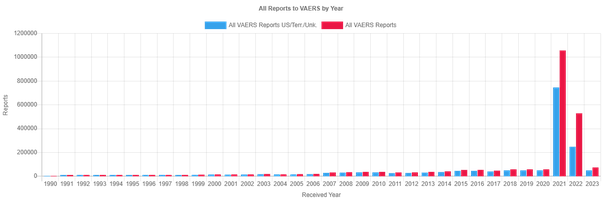
Something I’ve suspected for a while is that the risk of SAE or AESI are not evenly distributed across the country but varies depending on the vaccine lot. By how much I wasn’t sure but Dr. Craig Paardekooper analyzed the US VAERS database by batch number and found that only 1 in 200 are responsible for the vast majority of SAEs and deaths. Apparently 70% of the batches only produce one SAE while 80% only produce 2. For Pfizer in particular, who controls 70% of the market in the US and EU, just 3% of their lots account for 97% of deaths, 96% of hospitalizations and 95% of SAE reports.
Variation in Toxicity of Covid-19 Vaccine Batches
Completely unrelated Danish scientists replicated similar findings in Denmark published in the European Journal of Clinical Investigation for both the Pfizer and Moderna shots.
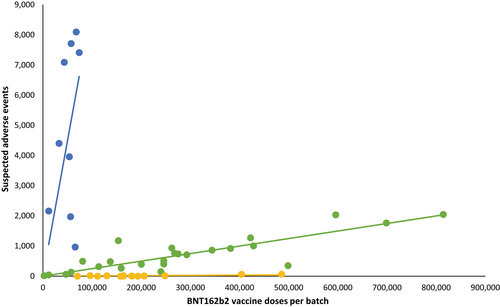
The larger batches (green and yellow) had lower rates of SAEs than smaller batches (blue). The Blue trendline represents only 4.22% of all doses but accounts for 71% of SAEs. While these two studies aren’t conclusive, they should add the growing suspicion of the quality control being done.
Let’s not forget that the BMJ revealed, through a FOIA request, that Pfraudster maintained two different manufacturing processes for 70% of their clinical batches (process 1) and 100% of their commercial batches (process 2).
Pfizer’s own safety report from December 2020 through June 2021 largely confirms the findings of Dr. Craig Paardekooper’s analysis of the VAERS database by batch number (Part 1) and the Schmeling analysis covered in (Part 2).
Just 20 batches account for 110,364 SAE cases out of 206,221 distinct cases.
See pages 50 through 57 of the Periodic Safety Update Report-1
This is perhaps the most vital revelation in this series as the source is a Reuters FOIA request of a routine FDA inspection of a Moderna modRNA production site conducted in late September. At the Massachusetts production site under inspection, the FDA found 5 separate quality control problems including (1) releasing 8 lots that were produced with equipment that had failed Moderna’s own cleaning verification test (2) storing more than 2,000 expired items in the same storage facility as items used to make the vaccine (3) using materials beyond their expiration date (4) not having protocols to ensure the final product was not exposed to airborne contaminants. Of course, Reuters is not interested in the truth so much as they are in sinking the reputation of Pfizer’s only other competitor in the modRNA shot market. While Moderna and the FDA claim there is no evidence of these low quality lots making it to market and being administered to the public following the precautionary principle would entail suspending the doses Moderna already produced as Japan did two years ago when they found contaminants in some vials within a lot of 1.6 million doses. There is no telling how long these quality control problems have been the norm there.
Potential Proteinopathy from Ribosomal Frame shifting
Last year an in vitro human and rodent study, published in Nature, discovered ribosomal frame-shifting during the translation of the modRNA that instructs your cells to synthesize the spike protein. As I briefly summarized in my prior answer, this mis-translation and subsequent production of an off target protein occurs as a consequence of replacing the nucleoside uridine with methyl-pseudouridine causing the ribosomes to stall and shift the protein sequence one or two bases. While the study did not find any adverse events in their small sample size and concluded that they were likely just junk proteins, it did pique the interest of a particular French Bio-mathematics lab who suspected a remote probability that some of the off-target proteins could be malignant and contain a prion region that could misfold other proteins in the body and cause diseases such as Creutzfeld-Jakob (CJD) which has been identified in 26 case studies following a modRNA shot. In their study, Perez and colleagues used a combination of the PLAAC algorithm to search for prion sequences in one and two base shifted spike sequences as well as the BLAST algorithm to search for other similarities with wild protein sequences. Unfortunately, what they found were not just junk proteins but a prion sequence in the one base shifted spike protein, which is not present in any circulating omicron variant but was in the ancestral variant, and a sequence that is found in the Naegleria fowleri species of amoebas commonly known as brain eating amoebas after two base shifts. While this study does not conclusively show that ribosomal frame shifting by one or two bases will cause these dire results it should be cause for concern and further investigation.
Immune Tolerance, Imprinting and Antibody Dependent Enhancement
Some of the adverse risks of modRNA are less age, sex, and lot specific and more platform and time specific. Immune tolerance, imprinting and antibody dependent enhancement (of disease) for immunocompromised recipients has only been observed following administration of modRNA booster but not their adeonviral vector (e.g. Oxford-AstraZeneca, Janssen & Sputnik V), full length recombinant spike protein (e.g. Novavax), or inactivated virus (Sinopharm & Sinovac). These risks are not only exclusive to the modRNA platform they also have only been observed a few months after a booster dose.
Not all antibodies are equally protective. Repeated modRNA doses has been found to promulgate an antibody subclass shift from more inflammatory IgG1, IgGE and IgG3 antibodies that stimulate complement immune responses such as phagocytosis of infected cells to a higher prevalence of non-neutralizing IgG4 antibodies that not only don’t stimulate complement immune processes but may actually block other sub classes of antibodies from doing so, resulting in disease enhancement, because the body typically creates them after repeated exposure to non-pathogenic antigen to induce immune tolerance [30]. IgG4 prevalence has been found to increase from 0.04% of antibodies after the primary series to 19.3% after the first booster dose. Valk et al., found that IgG4 levels rose as high as 21.2% after the first booster dose. However, this precipitous rise in IgG4 levels was only seen if the recipient was vaccinated prior to infection [31].
A recent blood sample study conducted by Kalkeri et al., which analyzed and compared antibodies from blood samples taken 6 months after patients had received their last Pfizer or Moderna boosters followed by a single dose of Novavax recombinant spike shot (n = 20) with patients who had received only 4 Novavax doses (n = 18) found that recipients of 4 Novavax recombinant spike had >10x higher anti-spike IgG3 (inflammatory) antibodies, which are responsible for 80% of neutralizing activity, compared to recipients of ModRNA boosters while said recipients had >75x anti-spike IgG4 (non-inflammatory) antibodies that are linked to immune tolerance and suppression.
The subclass shift takes at least a few months to occur which may explain why we observe initially high VE against infection and symptoms in the first two months that rapidly declines between months 2-3 post administration, becomes negligible between months 4-6 and results in negative VE against infection after 6 months (well before the next annual booster rollout). The evidence for this can be found in many observational studies:
A study in the Journal of the American Medical Association compared neutralization antibodies in blood samples from participants three months after the primary series (n = 73) and the first booster dose 10 weeks (2.5 months) post administration (n = 55). The study found that detectable geometric mean titers against omicron dropped from 76.2% four weeks after the booster, to 53.3% 8 weeks after the booster, to 18.9% 12–14 weeks after administration of the booster suggesting a rapid decline in omicron specific titers after only a few weeks.
Wang et al., found that there are no discernible differences in omicron neutralization between the older monovalent (used in the study above) and the new bivalent:
‘Boosting with a new bivalent mRNA vaccine targeting both BA.4/BA.5 and an ancestral SARS-CoV-2 strain did not elicit a discernibly superior virus-neutralizing antibody responses compared boosting with an original monovalent vaccine.’
After analyzing serum collected from individuals who received 3 doses of the monovalent and comparing them to those who received the bivalent (an average of 26 and 24 days after vaccination). All participants exhibited the highest neutralizing titers against the ancestral variant and there were no statistically significant differences in the neutralization of any variant between the groups 3–5 weeks after the booster.
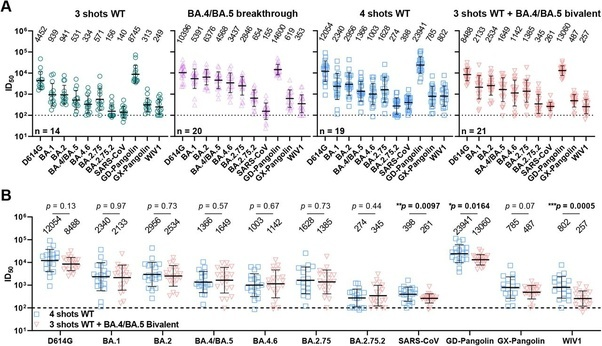
A study published in the New England Journal of Medicine (Davis-Gardner et. al.,) also analyzed serum samples from (n = 35) participants: 7–28 days after the first monovalent booster (n = 11), 6–57 days after the second monovalent booster, and 16–42 days after the bivalent booster (n = 12). The study found substantially lower neutralization activity against all omicron subvariants compared to the wildtype-ancestral variant in all three cohorts. In both monovalent cohorts (n=23)(Geometric Means) antibody titers against BA1 and BA5 were 5–9x lower than against wildtype-ancestral variant and 23-63x lower against BA.2.75.2, BQ1.1 and XBB than the wildtype-ancestral variant. In the bivalent booster cohort, (Geometric Means) antibody titers against BA1 and BA5 were 4x lower than against wildtype-ancestral variant and 12–26x lower against BA.2.75.2, BQ1.1 and XBB than the wildtype-ancestral variant.
Another study published in the New England Journal of Medicine (n = 33) found that both the monovalent (n = 15) and bivalent (n = 18) boosters lead to the expansion of antibody titers for the wildtype-ancestral variant and lower antibody titers for the BA5 omicron variant after a median of 3 doses. Memory T cell responses were barley affected in either group and the bivalent booster produced a slightly higher level of antibody titers at a rate ratio of 1.3.
Another Study published in the New England Journal of Medicine (Hachmann et al.,) confirmed that Pfizer booster produced a much more robust antibody response to wildtype-ancestral variant, with a median 5,783 antibody titers 2 weeks after administration, and a much more subdued response to the omicron subvariants with a median 900 antibody titers against BA1, median 829 antibody titers against BA2, median 410 antibody titers against BA.2.12.1, and a median 275 antibody titers against BA4 and 5 2 weeks after the booster. All but one of the participants infected with BA1 and 2 subvariants were vaccinated suggest substantial immune evasion by omicron subvariants from the booster immunization. Participants with prior infections had median antibody titers of 11,050 against wildtype-ancestral variant, 1,740 against BA1, 1,910 against BA2, 1,150 against BA.2.12.1, and 590 against BA4 and 5.

Another study published in Vaccines, (Sheehan et al.,) collected blood samples from (n = 16) SARS-COV-2 naive booster recipients at 6 times points over 420 days, including prior to vaccination, and found that receptor binding domain and spike-specific IgG4 antibody levels were significantly elevated in boosted but not primary series immune blood samples. IgG1 and IgG3 neutralizing antibody levels peaked at 3 weeks after the second dose and declined after four months. While IgG2 and IgG4 levels were initially negligible during the primary series the booster dose induced changes in the subclass distribution. While receptor binding domain and s-protein reactive IgG2 and IgG3 were not detected 6 months after the booster antigen specific IgG1 and IgG4 antibodies persisted after this duration. Neutralizing titers declined to pre-immune levels 6 months after the booster.
While boosters enhanced serum IgG Ab reactivity and nAb responses against variant strains, all variants tested showed resistance to two- and three-dose immune sera. Our data reflect the poor durability of vaccine-induced nAb responses which are a strong predictor of protection from symptomatic SARS-CoV-2 infection. The induction of IgG4-switched humoral responses may permit extended viral persistence via the downregulation of Fc-mediated effector functions.
A rodent study published in iScience additionally finds that repeated boosters that target the receptor binding domain impairs serum neutralization, the activation of T memory cells, and leads to immune tolerance. Immune tolerance is the body’s inability to produce antigen specific antibodies that activate memory T cells or insufficient production of them upon encountering a new infection. The study detected a 2.5–4x reduction in average antibody titers against the delta and omicron variants compared to the wild type and a (statistically) significant reduction in the serum neutralization in the extended (boosted) group against all 3 types compared the the conventional (primary series) group.
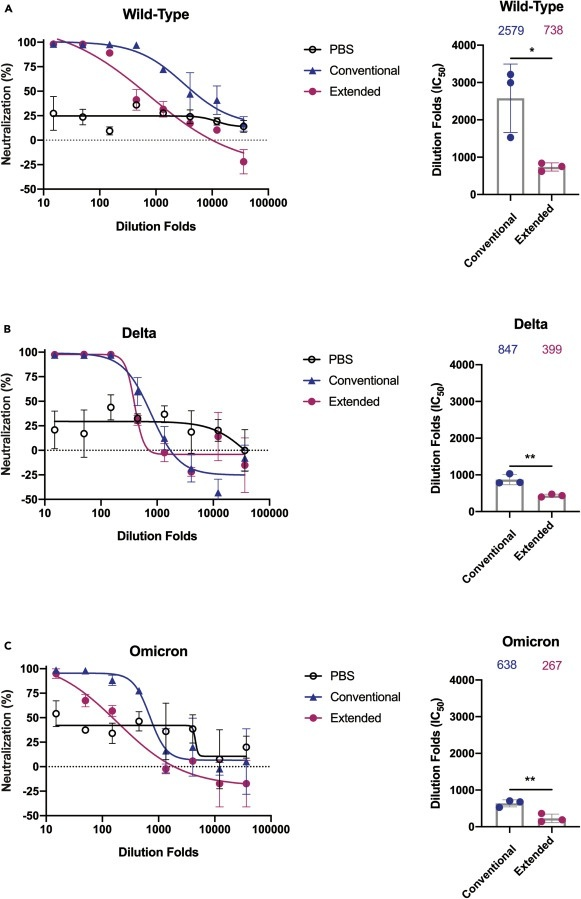
Note: PBS is the control group.
XBB1.5 Booster is completely useless against the dominant JN1 subvariant.
A recent antibody study published in The Lancet analyzed neutralization activity against newly emerging subvariant BA2.86 in blood samples taken from monovalent and bivalent boosted participants found that BA.26 evades booster induced humoral immunity. The three monoclonal antibodies that worked against the parental BA2 parental subvariant had no effect against BA.26 infections have been detected in 11 countries and the subvariant’s spike protein is 30 mutations separated from the parental BA2 subvariant. BA2.86 reproductive rate is 1.3x higher than XBB1.5 and similar to the reproductive rate of EG5.
A rodent study, also published in The Lancet, found that BA2.86 (and its sub lineage) is antigenically distinct enough from XBB.1.5 (the updated booster) and prior omicron sub variants that it can evade XBB induced nABs. Using a psuedovirus, they found that BA2.86 was resistant to serum nab titer levels in mice injected with two doses of spike ModRNA shots against B1, BA.5, BQ.1.1, and XBB. Using a psuedovirus neutralizing assessment, they found that BA2.86 was just as evasive against nabs levels in two cohorts of mice (n = 81) inoculated with 3 inactivated COVID vaccines that had either a XBB breakthrough infection or an XBB reinfection after BA.5 or BF.7 breakthrough infection.
Another study, published in the Lancet, that collected human blood samples from participants that had received the most recent XBB1.5 monovalent dividing them into cohorts without prior SARS-2 infections (n = 9) and those with a prior XBB sub-variant infection prior to the booster (n = 10) found that some individuals in the former cohort had no anti-viral activity against: XBB.1.5 (n=2), XBB.1.16 (n=1), XBB.2.3 (n=3), EG.5.1 (n=3), HK.3 (n=3), and BA.2.86 (n=2) 20–29 days after their booster dose.
A combined cell culture and serological French study (n = 75), published in Nature, found that 3 monovalent doses (primary series + first booster) is completely worthless against the current dominant variant (JN1) and dominate variant from the prior year (XBB1.5) with no detectable neutralization activity against the recent XBB-derived or BA.2.86 variants. The 2022 Bivalent booster triggered a moderate and brief neutralizing antibody titer levels against XBB and BA2.86 derived variants with barely detectable neutralization activity after 6 months while XBB infections triggered a broader cross-neutralizing response than bivalent boosters and reduced antibody titer level differences between subvariants XBB.1, EG.5.1 and BA.2.86.1. The study also uncovered evidence of probable immune imprinting from repeated boosters finding that the bivalent produced the highest antibody titer levels against the ancestral variant, a 6–9x lower titer level against BA1 and BA5 and 10–25x lower antibody titer levels against XBB and BA2.86 derived subvariants compared to BA1 and BA5
Veesler and colleagues, publishing in Cell, discovered definite evidence of immune imprinting from a handful of peripheral blood samples collected at two different times from recipients of the XBB1.5 modRNA booster up to 2 months post administration tested against a vesicular stomatitis virus psuedotype with multiple variants ranging from the ancestral variant to the current dominate variant JN1. They found that only 5 of the 12 participants profiled 10 days post booster had memory B cells that recognized the XBB1.5 receptor binding domain alone without cross reacting to the ancestral variant at rare frequencies Only 3 out of 9 recipients profiled 51 days after the booster had memory B cells that recognized the XBB1.5 receptor binding domain alone without cross reacting to the ancestral variant and only 2 out of 7 recipients that provided samples at both time points had XBB1.5 specific memory B cells that didn’t cross react to the ancestral variant. Antibody titers elicited against the ancestral variant were much higher than they were against XBB1.5 which was also much higher than it was against JN1.
Another yet to be published serum study that utilized psuedoviruses presenting the entire mutational spectrum of the SARS-COV-2 genome over the past four years (n = 20) found that JN1 was significantly resistant to serum neutralization across all doses including recipients of the latest XBB1.5 booster. JN1 and the beta variant from 3 years ago escaped serum neutralization of the XBB1.5 booster.
Side Note: Observations of modRNA shots creating immune tolerance to specific antigens and possible enhancement of disease are not new and have previously been observed in clinical trials for HIV [113] and Malaria vaccines [115] which directly linked increased risk of infection with IgG4 selection.
Nature Microbiology identifies non-neutralizing and sub-neutralizing antibodies (e.g. IgG2 & IgG4) binding to viral antigens as one of two mechanisms that creates antibody dependent enhancement, specifically by binding to viral surfaces and incorporating them into white blood cells that effectively become hosts for replication. Non-neutralizing antibodies have been found to enhance viral infections of alveolar and peritoneal white blood cells in particular.
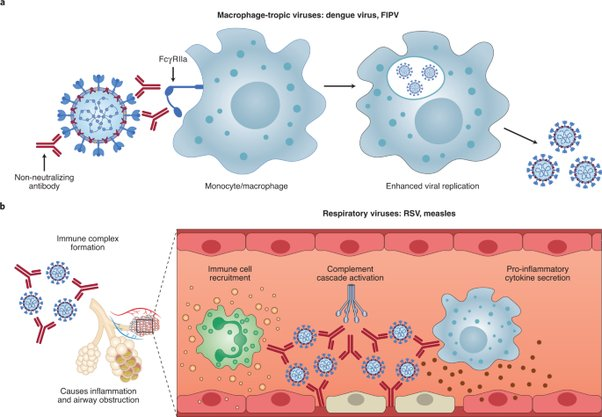
An autopsy study published in Nature (n = 27), found that white blood cells are infected at a higher rate than lineage tagged smooth muscle derived cells. The vitro experiment also found that the virus is able to replicate in both plaque white blood cells and cholesterol containing white blood cells called foam cells. The vitro experiment further found that the virus preferentially replicates in the cholesterol containing “foam cells” compared to other white blood cells of the immune system.