Insulators and Conductors
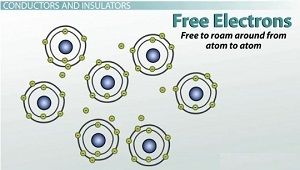
Free electrons are free to roam from atom to atom
Back in the old days, if someone's house caught on fire, volunteers would rush to the scene and form a bucket brigade to put out the flames. If you think about it, you could say that the bucket brigade was 'conducting' water from the source to the fire. But what if the volunteers stopped passing the water?
Well, we know one thing: the house would burn to the ground because it was effectively 'insulated' from the water!
Not all atoms are created equal. Some atoms don't hold on to their outer electrons very tightly. These are known as free electrons because they are literally free to roam around from atom to atom. Free electrons are the members of our electrical bucket brigade, passing electrical energy from one electron to another.
A material with many free electrons allows easy transfer of electrical energy and is therefore called aconductor. If we send an energetic electron into a conductor, it will impact a free electron, knocking it down the line until it hits another free electron. This sets up a chain reaction of impacts that conducts the electrical energy through the material.
A good way to think of it is like a group of balls spread out on a billiards table. Our energetic electron is like the cue ball being shot into the group and impacting one ball, which in turn knocks into another ball and so on down the line. Before you know it, the energy from the cue ball has been 'conducted' all the way to the other end of the table. The only real difference between billiard balls and electrons is that electrons conduct electrical energy at nearly the speed of light!
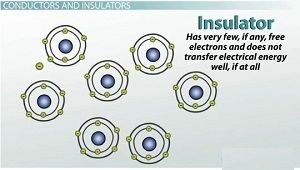
Insulators have very few free electrons and do not transfer electrical energy well
On the other end of the spectrum, there are atoms that hold on to their electrons very tightly. A material that contains these types of atoms has very few, if any, free electrons and does not transfer electrical energy well, if at all. This type of material is called an insulator.
If we send an energetic electron into an insulator, it effectively bounces off the atoms, unable to transfer its energy to the tightly bound electrons. It will keep bouncing around until it either frees another electron or until it simply runs out of energy!
Going back to our billiard table analogy, this is very similar to the cue ball simply bouncing off the sides of the table. Either it will hit another ball and transfer its energy, or it will just stop rolling because of friction.
Conductivity
The ability of a material to conduct electrical energy is known as its conductivity. The conductivity of a material is dependent on the number of free electrons available, and this varies greatly between the different types of atoms. In general, the materials with the highest conductivities, or best conductors, are metals - but this doesn't mean that other materials aren't capable of conducting electricity. If that were true, no one would ever be in danger of getting electrocuted!
Examples of materials with low conductivities
Not all materials are classified as insulators or conductors, because in the real world, they don't do a particularly good job of either one.
Final note on conductivity: good electrical conductors usually good heat conductors. Electricity and heat are just two different forms of energy, but both rely on free electrons to transfer through the material. Sometimes it's easier to tell if a material would be a good conductor by using our sense of touch to see how well it conducts heat.
good post.keep it up