Ab Inito Chemistry Simulation
Chemistry is A bit off my normal path of interest, but computer simulation - that's right up my alley. Take, for instance, an interesting molecule like
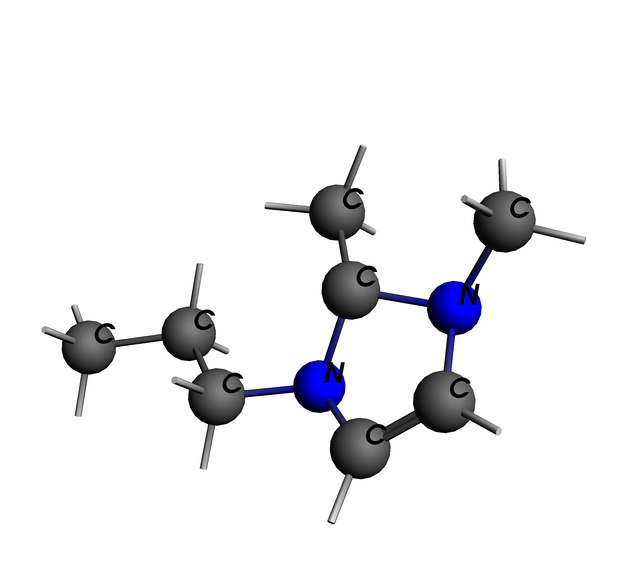
The 1,2-Dimethyl-3-propylimidazolium Cation
This is an ionic liquid component; you'd pair it with an Anion like Iodide and go to town. Doing what, you say? Well, They can dissolve gasses - and when they do, there should be chemical changes to them. Plus they have low vapor pressure, compared to water or alchohol. So, you should be able to set up sensors for all sorts of things using Ionic Liquids... CO2, H2, NO-, Methane, Freon - lots of different stuff. And if you choose your Ionic Liquids right, you have differentiation and specificity, so you sense only what you want.
So, because of the stability and nonvolatility, you get a chemical sensor which can last a long time, and give you a good readout, without all that mucking about with a chemical cell and expensive electrodes. All you have to do is find the correct combination of Anion and Cation to react with exactly what you want. But with hundreds of Cations and dozens of Anions, finding the needle in a haystack would be a time consuming and error-prone. This is where computational chemistry enters the scene. A low fidelity computation of state might take a few minutes per combination, But by comparing the state energy between the ionic liquid alone and the ionic liquid with the dissolved molecule of interest, you can decide whether you have a signal, and what type of signal it is. Then, you can do experiments with spoof chemicals, comparative concentrations, and so on - and have a good idea of which ionic liquids to start with for your sensor application.
Ref: Gas Solubility in Ionic Liquids Zhigang Lei, Chengna Dai, and Biaohua Chen, http://dx.doi.org/10.1021/cr300497a | Chem. Rev. 2014, 114, 1289−1326