FDA Has Approved New Drug for Traveler's Diarrhea

About 40 percent of travelers are affected by Traveler's Diarrhea around the world. If you have suffered with this condition, then you definitely know that it can ruin a vacation! But now, the FDA has recently signed off on a new drug that could alleviate the symptoms associated with the condition.

The new antibacterial drug has been named "Aemcolo" and will fight off symptoms caused by E. coli. It is predicted to be released to the US in Feb 2019. Users can take the drug orally for three or four days to see the best results, and it can be used before or during your vacation.

Aemcolo is said to be the first antibiotic for Traveler's Diarrhea that the FDA has approved in over 10 years! Side effects commonly seen were headache and constipation.
Traveler's Diarrhea is the result of pathogens and bacteria that get consumed through food and water. The high risk locations where one may experience this condition are parts of Asia, the Middle East, Africa, Mexico, and Central and South America.
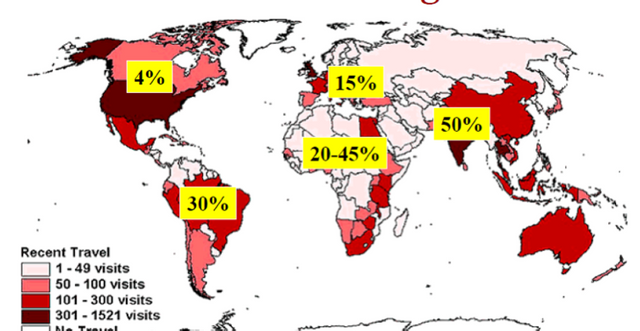
It has been noted that Aemcolo will not be effective in cases where diarrhea is caused by pathogens other than E. coli and results in fever or blood in the stool.

**None of the photos above are my original content.
https://www.travelandleisure.com/travel-news/aemcolo-new-traveler-diarrhea-medicine