¿Do you know the reason why your pan bends when it's hot and gets water poured on it?
• First I wish all of you, steemians; they are well and that they receive a cordial greeting! I also invite you to read my publications, they will always be welcome, I am sure that they will not get bored and they will be able to learn a little about some things.
• In this opportunity I will talk about a specific topic or rather a curious fact that I learned in the university, specifically in the Materials Science class, since this subject belongs to the Industrial Engineering curriculum which is the career that I am currently studying.

• We all know what will happen if when removing a hot pan from the burner we try to wash it immediately with cold water or water at room temperature; What if! This may be deformed, it all depends on the temperature in which this kitchen utensil is located and the liquid spilled on it, as well as the material that the pan is made of; but this has a scientific explanation and is that iron molecularly has a property of belonging to two types of crystalline structures.
o And what are the crystalline structures? it is simply the way in which the atoms of a material are ordered in order to form it.
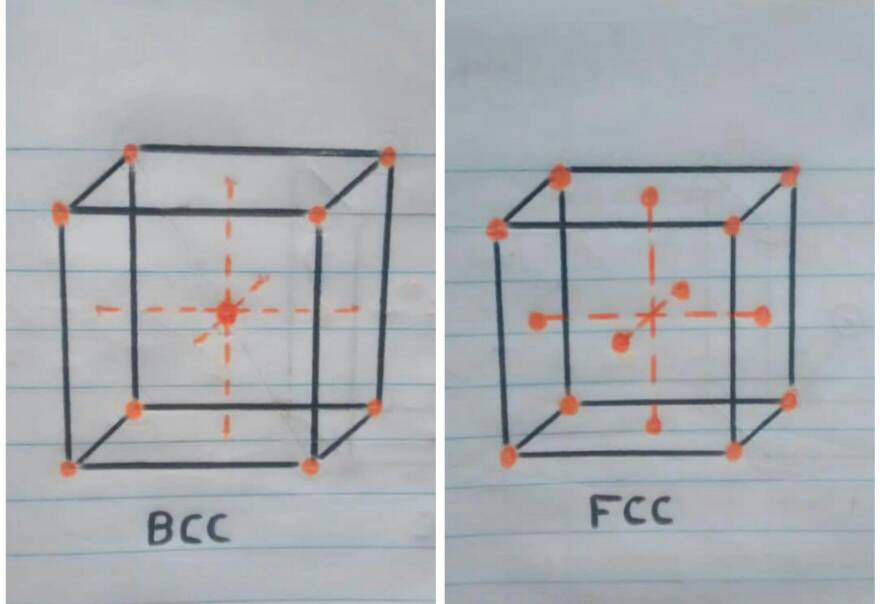
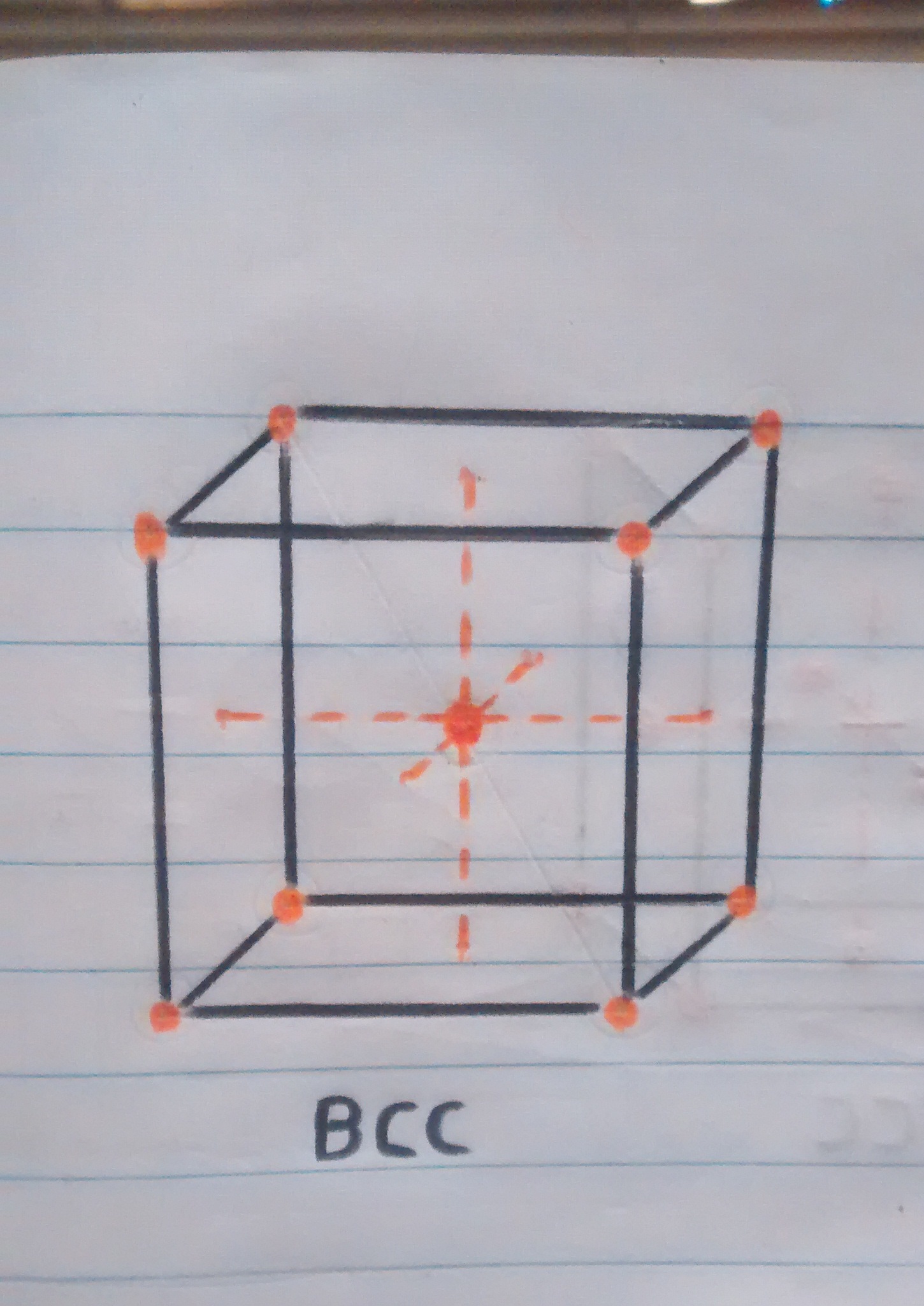
• Crystal structure at room temperature: when the iron is at room temperature the type of crystal structure is BCC (Cubic centered in the body).
• Crystal structure at more than 910ºc: once the iron reaches the temperature of 910ºc iron atoms are dispersed to the faces of the cube, automatically changing the crystal structure to FCC (Cubic centered on the faces).
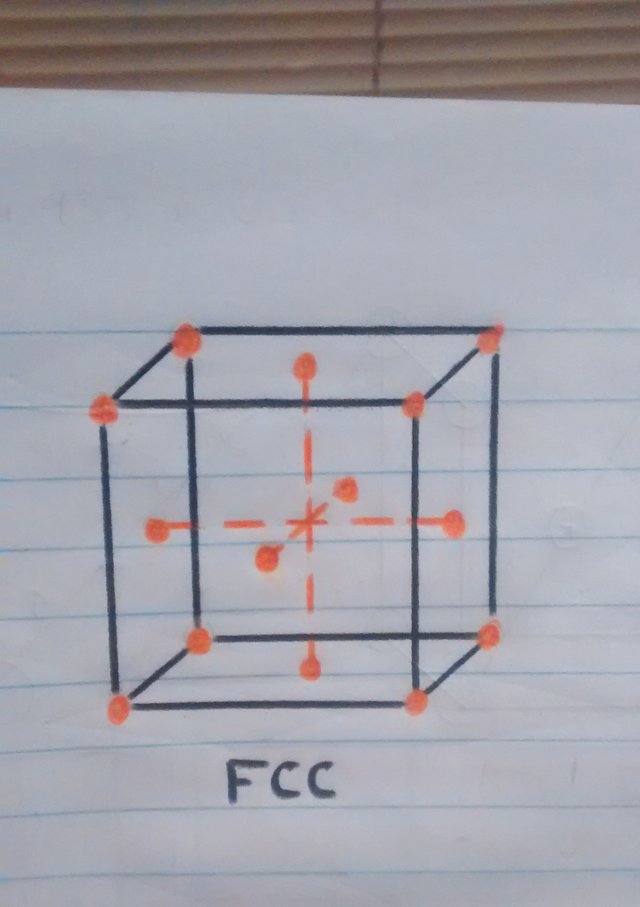
o But what is this bucket that is being talked about so much? this cube represents a minimum part of the material which is called a unit cubic cell and is seen in this way because the material is molecularly composed of millions of these cubes.
o Why is it that iron can belong to two crystalline structures? This is given due to a property that some materials have called Alotropy, which allows the material to pass from one crystalline structure to another, depending on the temperature and pressure. This process is similar to the reaction that occurs in the change from a liquid to a gaseous state.

• Now that we know a little more about crystalline structures, atoms and cubic cells, we can easily explain what happens when the pan is folded. When the temperature is reaching 910ºc the transition from one structure to another begins, resulting in the iron atoms of the pan that were in the center of the BCC crystal structure (the sphere in the center of the cube in figure 1). to move towards the faces or walls of the FCC cube, thus having the iron expand at these high temperatures.
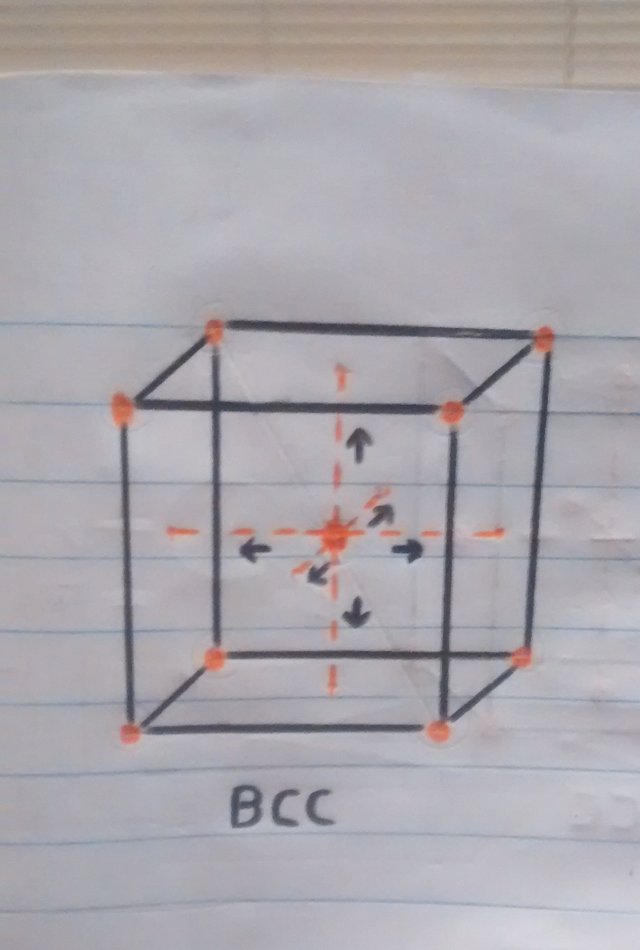
o Movement of atoms from the center towards the faces of the cube.
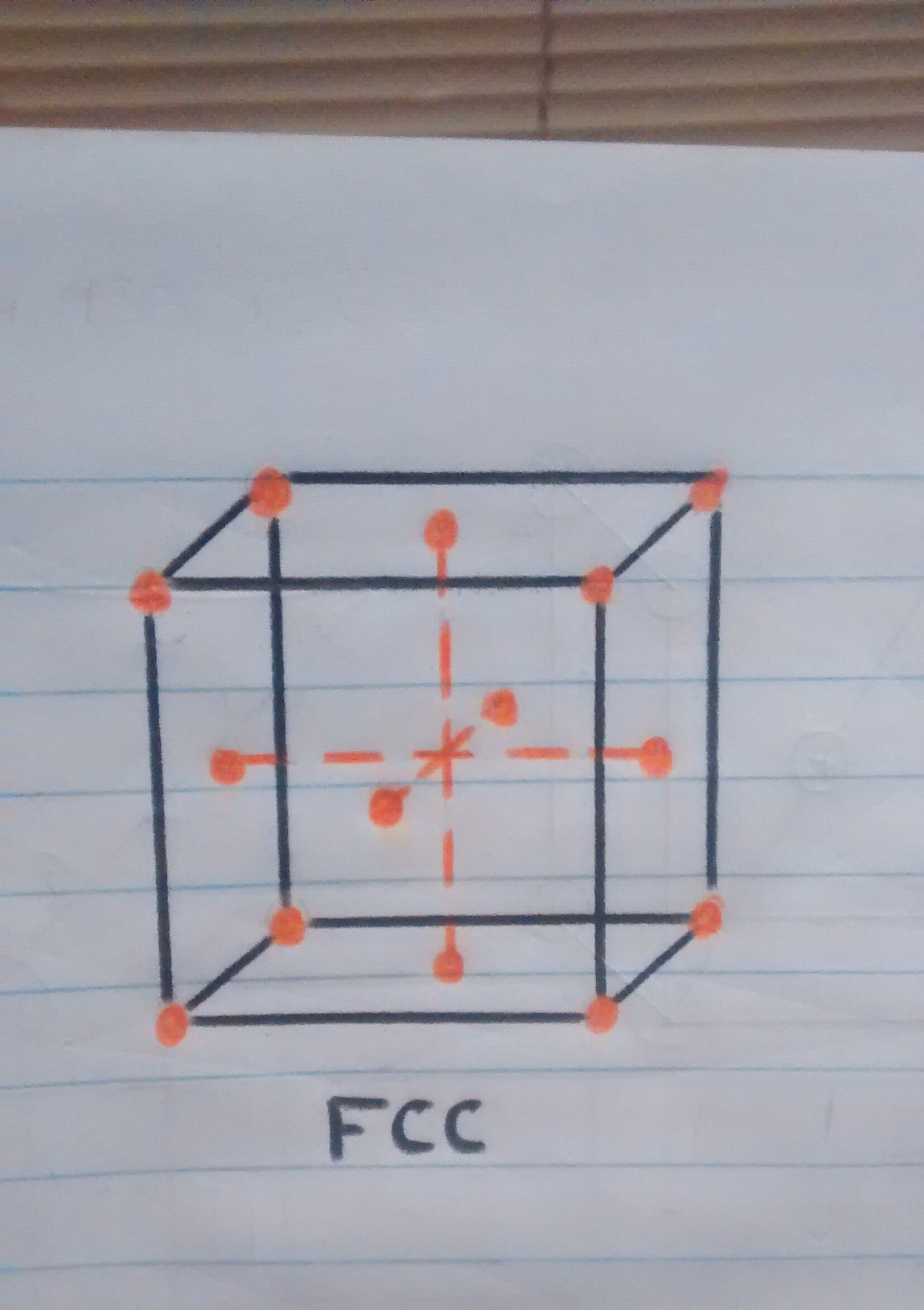
o Atoms on the cube faces after having changed crystal structure.
• Now, although we know that the material at some point will have to cool, this should do it naturally waiting for the atoms that are on the faces or walls of the cube to slowly regroup in the center of the cube since the process of cooling is not so fast, but if we subject the material to a sudden change in temperature by pouring cold water, the atoms that were moving towards the center of the cube will be petrified in the place they are, regardless of whether it is in the center since the transition between one crystalline structure and the other was interrupted, which would cause a disorder of the atoms in the structure of the material and that this can not be adequately contracted which would result in deformation of the pan and once in this state, unfortunately not there will be a return.
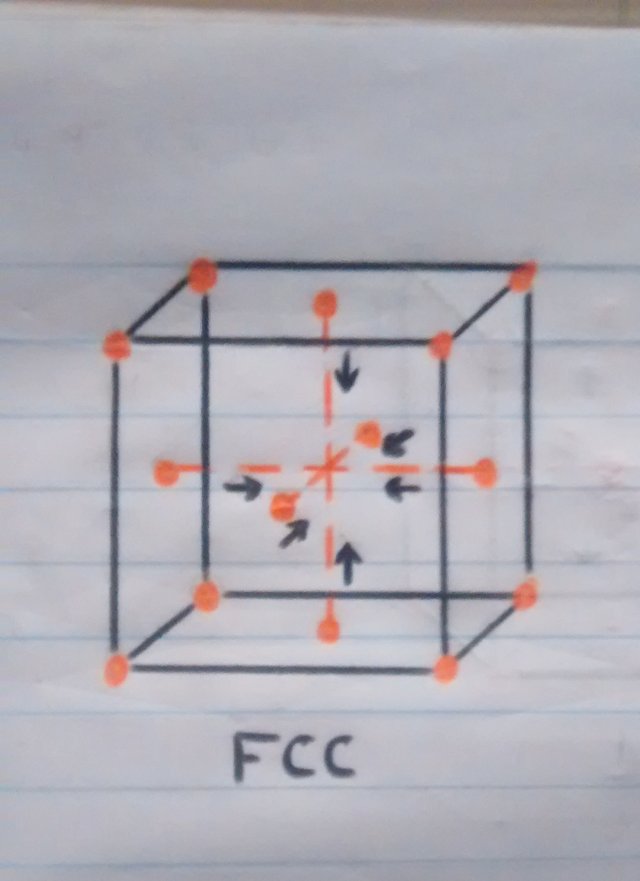
o Atoms returning to their main position while the material is cooling.

o Atoms in their original position while the material is cold.
o There are pans that fold more easily, it all depends on the percentage of carbon contained in the iron, the type of pan you have in your house, if the layer of the material is very thin and a series of other features that can contribute to this Deformed or cracked.
o I recommend you always let it cool before putting it in soak and then wash it hahaha XD, I say this from my own experience.
o Thank you very much for reading this information, I hope it could have been of great interest to you and that you have not got bored with all the information.
o All the drawings were made by me, there was no source of information that was alien to the one I already had in my notes and my knowledge.
o If you have any suggestions or any constructive criticism that can help me to make this adventure in @steemit more incredible, I would be very grateful.
o It would be great if you follow me and you vote in my post, I will thank you very much.
o Source: all the drawings were made by me, there was no source of information that was alien to the one I already had in my notes and my knowledge.
o Steemit: @tuaprendizaje
Hello @tuaprendizaje!
I noticed you have posted many times since you began your journey on Steemit. That is great! We love active partipants.
I do want to point out that the Introduceyourself tag is meant to be used once only to introduce yourself to the Steemit community. You have now posted 5 times using the introduceyourself tag. Please see this link for more information on Tag Spam?
Please take this into consideration and help build a great platform!
Welcome to Steemit @tuaprendizaje :)
Welcome to steemit @tuaprendizaje. Join @minnowsupport project for more help. Checkout @helpie and @qurator projects.
Send SBD/STEEM to @treeplanter to plant trees and get an get an upvote in exchange of your donation (Min 0.01 SDB)
Upvote this comment to keep helping more new steemians
Send SBD/STEEM to @tuanis in exchange of an upvote and support this project, follow for random votes.