Atmospheric pressure: unit conversion table
Hi, friends of Steemit!
This time I present a brief definition of atmospheric pressure, characteristics and annex a conversion table that I made myself some time ago, in paint, plu some conversion exercises and to find the atmospheric pressure at a certain latitude and altitude.
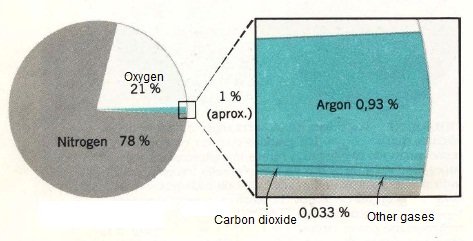
The atmosphere is composed of various gases, which vary in proportion according to altitude and latitude, or by the weather in a given time. In the case of the troposphere, where we live, the proportions of these gases are:
| Composition | Percentage (%) |
| Nitrogen (N2) | 78,08 % |
| Oxygen (O2) | 20.95 % |
| Argon (Ar) | 0.93 % |
| Water vapor | 2 % Surface |
What is Atmospheric Pressure?
It is Pressure exerted by the atmosphere per unit area. This means that the atmosphere press us constantly, but why do not we feel it? due to the fact that the gases apply force in all directions. The density of these gases depends on the pressure, and the altitude, as observed in the following table, the density decreases with the height:
| Height above sea level (Km) | Density of the air (kg/m3) |
| 0 km | 1.23 |
| 10 km | 0.41 |
| 20 km | 0.09 |
| 30 km | 0.01 |
Characteristics of atmospheric pressure:
-It is considered normal pressure (Standard), when the pressure is equal to 1013.25 mb at sea level, at 0 ° c and at 45 ° latitude.
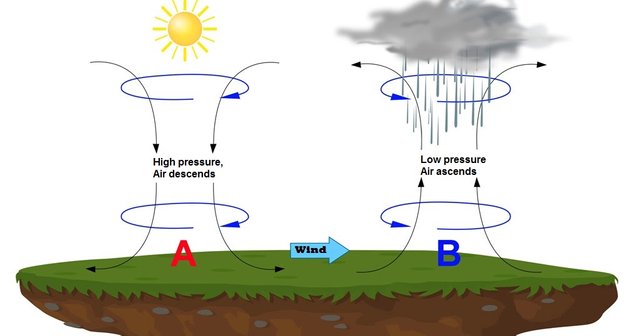
-High-pressure zone: They are considered when their values are bigger than 1020 mb. The high pressure zones are known as anti cyclone, because they are associated with good weather, clear or sunny.
-Low-pressure zone: They are considered when their values are less than 1013 mb, these zones are known as cyclone or cyclonic zones. Therefore they are associated with disturbances in the atmosphere, disturbances or bad weather.
Variation of atmospheric pressure
-Variation by heating: Decreases the density
-Variation by cooling: Increases density.
-The pressure has a very small horizontal variation, compared to the vertical variation, it varies approximately every 111 km of horizontal distance.
-Variation by height: The higher the elevation, the lower the pressure.
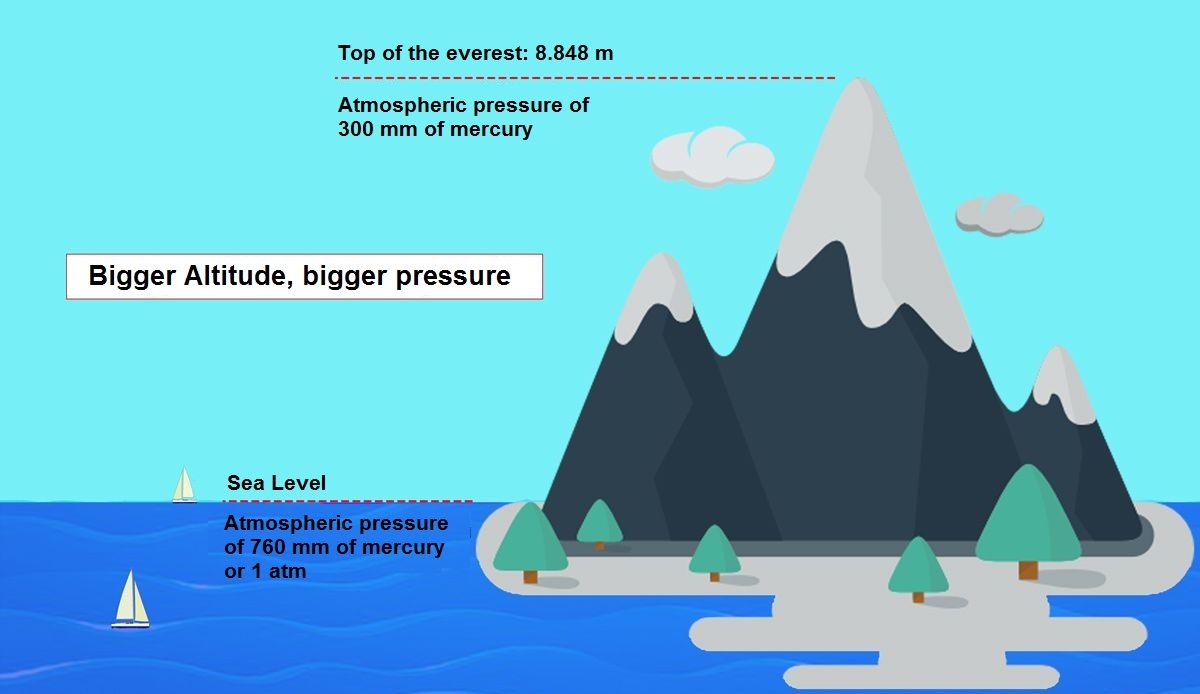
-Variation per day by latitude, see table:
| Latitudinal zone | Daily variation (mb) |
| Tropic | 3-4 mb |
| 23,27° - 50° | Aprox 1,3 mb |
| 50° L | 0,9 mb |
| 60° L | 0.4 mb |
| Bigger latitude | Almost inappreciable |
As we can see in the previous table, the pressure fluctuates much more in the equator and its variation decreases each time we get closer to the poles.
What are the components of the atmosphere that exert the bigger pressure?
| Nitrogen | 750 mb |
| Oxygen | 230 mb |
| water vapor | 10 mb |
How calculate the atmospheric pressure:
Pressure = Force / Surface Unit
Hydrostatic pressure= Height x specific gravity
Standard Pressure= Normal atmosphere x (T° - K x h / T°)5.256
; Where:Normal atmosphere= 1013.25 mb - T° in degrees Kelvin (c° + 273) - K= 0.0065 (meters) o 6,5 (kilometers)
Example of standard pressure:
We have the next data:
- Altitude= 1 Kilometers
- T°= 15,15 °c (We take her to Kelvin Grades adding 273 = 288,15 °K)
We apply the calculation:
P= 1013.25 mb (288,15 °K - (1 km x 6,5 km) / 288,15 °K)5.256 = 898,74 mb
898,74 mb, is the standard pressure in the place, at 1 km altitude and 15,15 °c
To obtain the result, we eliminate (km with km) and (° k with ° k and obtain the value in millibars (mb)
Conversion table of atmospheric pressure units
There are diverse pressure units, the first were those used by torricelli, over time others have appeared to simplify the calculations, the next table was made by me, months ago, in the course of meteorology that i witnessed:
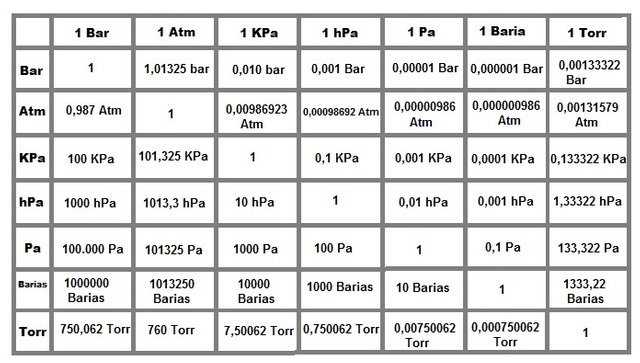
| Unit | Abbreviation |
| Bar | Bar |
| Atmosphere | Atm |
| Kilopascal | kPa |
| Hectopascal | hPa |
| Pascal | Pa |
| Barias | Barias |
| Torricelli | Torr |
We can observe that i order the units in a way that is easy to convert through the famous "rule of 3"
Example:
- Convert 10 Atm to mb, we simply make this relationship:
| 1 atm | = | 1013,3 mb |
| 10 atm | = | ? |
Then: mb= 10 atm x 1013,3 mb /1 atm= 10133 mb
Eliminate the atm with atm and we have left in mb.
- Convert 10 torricelli to pascal (Pa)
| 1 Torr | = | 133,322 Pa |
| 10 Torr | = | ? |
Then: Pa= 10 torr x 133,322 Pa / 1 Torr = 1333,22 Pa
We eliminated Torricelli with Torricelli and we have the result in Pascal (Pa)
In conclusion take into account that:
1 hectopascal (hPa) it's the same as saying 1 milibar (mb) = 1hpa = 1mb
1 torricelli (Torr) has a very minimal difference with a 1 mmHg = 1 Torr=1mmHg

I hope that the data of this table, if you observe any error, in quantity or by approximation, please, tell me to correct. Thank you very much, regards, friends!
Sources:
- Guevara Díaz José Manuel (1988) Meteorología
- Arthur Strahler y Alan Strahler (1952) Geografía Física
- Wikipedia.org
- Lessons learned in the meteorology course at the geography school - Universidad de los Andes, Mérida, Venezuela
This publication has registered to the writing contest thanks to @apple96.
writing contest thanks to @apple96.
For more information, click here!!!!
See the Minnowhelper contest conditions here
You received a 10.0% upvote since you are not yet a member of geopolis and wrote in the category of "geography".
To read more about us and what we do, click here.
https://steemit.com/geopolis/@geopolis/geopolis-the-community-for-global-sciences-update-4
Your Post Has Been Featured on @Resteemable!
Feature any Steemit post using resteemit.com!
How It Works:
1. Take Any Steemit URL
2. Erase
https://3. Type
reGet Featured Instantly & Featured Posts are voted every 2.4hrs
Join the Curation Team Here | Vote Resteemable for Witness