Which is more effective in preventing COVID-19, Ivermectin or the COVID vaccine?
The booster is recorded as 21% effective against infection for covid naïve patients and 33% effective against infections for patients who acquired natural immunity according to a retrospective matched cohort study conduct in Qatar (n = 68,288). The Cleveland Clinic study, another matched cohort study, (n = 51,011) recorded a similar result of 30% effectiveness against new infections but found a higher reinfection rate among recipients of the new booster compared to colleagues with 2–3 shots and cited three other studies, most are behind paywalls, that found an inverse relationship between number of shots and reinfection rate.
This is not the only study to find a possible association with more prior vaccine doses and higher risk of COVID-19. A large study found that those who had an Omicron variant infection after previously receiving three doses of vaccine had a higher risk of reinfection than those who had an Omicron variant infection after previously receiving two doses of vaccine [21]. Another study found that receipt of two or three doses of a mRNA vaccine following prior COVID-19 was associated with a higher risk of reinfection than receipt of a single dose
Another retrospective matched cohort study conducted in Qatar (n = 2.28 million), and published in The Lancet, found that the first booster (third dose) is about 26.2% effective against omicron infection for recipients with no prior infection, 25% effective for recipients with a pre-omicron infection, and 22% effective for recipients with a previous omicron infection. The study also found evidence for negative immune imprinting/vaccine dependent enhancement; initial protection against infection was assumed to be high in the first month of immunization, well above 50%, but quickly dropped to 8.3-15.5% 6 months later and became negative, meaning recipients were more prone to reinfection than their 2 dose counterparts, in the 7th month.
At least one of them was not and was published in the New England Journal of Medicine finding that immunity conferred from prior infection was superior to two doses of the primary series against omicron:
The number of cases of SARS-CoV-2 infection per 100,000 person-days at risk (adjusted rate) increased with the time that had elapsed since vaccination with BNT162b2 or since previous infection. Among unvaccinated persons who had recovered from infection, this rate increased from 10.5 among those who had been infected 4 to less than 6 months previously to 30.2 among those who had been infected 1 year or more previously. Among persons who had received a single dose of vaccine after previous infection, the adjusted rate was low (3.7) among those who had been vaccinated less than 2 months previously but increased to 11.6 among those who had been vaccinated at least 6 months previously. Among previously uninfected persons who had received two doses of vaccine, the adjusted rate increased from 21.1 among those who had been vaccinated less than 2 months previously to 88.9 among those who had been vaccinated at least 6 months previously.
Among persons who had been previously infected with SARS-CoV-2 (regardless of whether they had received any dose of vaccine or whether they had received one dose before or after infection), protection against reinfection decreased as the time increased since the last immunity-conferring event; however, this protection was higher than that conferred after the same time had elapsed since receipt of a second dose of vaccine among previously uninfected persons. A single dose of vaccine after infection reinforced protection against reinfection.
Moderna’s own phase 3 trial data for the booster recorded a higher infection rate among the new boosted (3.2%) compared to participants who had only received the old booster (1.9%): a 68% higher relative risk of infection after receiving the new booster.
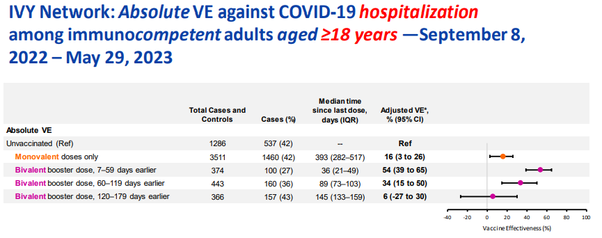
A CDC report found that absolute “vaccine” effectiveness against hospitalization after the monovalent primary series (median 464 days post shot) is -8%. That means, the jabbed are more likely to be hospitalized than the unjabbed. Yet, those jabbed with bivalent boosters median 137 days prior reach the same -8% “vaccine” “effectiveness” as those jabbed a year and a half earlier with “regular” Covid-19 jabs against the latest Covid XBB variant:
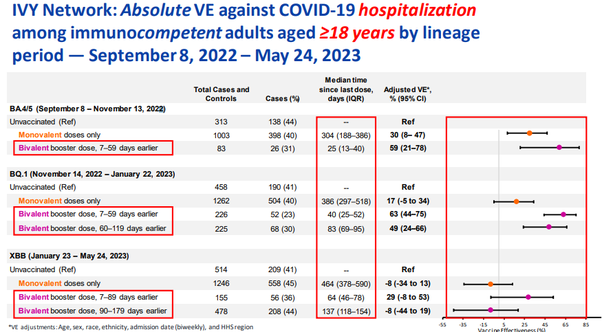
The VE against ANY Covid variant is much worse for a recent 145 days prior bivalent booster than for a 393 days prior last monovalent shot and rapidly approaches the negative territory:
A Pfizer sponsored test-negative case-control study, published in the Journal of the American Medical Association, of children (n =24,261) between 6 months to 4 years of age found an overall VE of 33% against symptomatic infection for the combined two and three shot group but a higher risk of symptomatic infection after three shots compared to two shots within the 90 days window of the study. The three-shot group VE against symptomatic infection was only 12% compared to the unvaccinated group (i.e. within the confidence interval and basically negligible) within the 90 days window of the study. It should be remembered that three 3 microgram doses is considered a standard for children and only 2 is considered an incomplete series; thus, completely vaccinated children between 6 months and 4 years of age have negligible protection compared to unvaccinated children.
A recent CDC vaccine effectiveness study (n = 7,434) that enrolled children who received medical care for acute respiratory illnesses, between 6 months and 4 years of age, through the New Vaccine Surveillance Network, found that 28.5% (n = 1,816) of unvaccinated children (n = 6,337) in the study required supplemental oxygen compared to 35.23% (n = 99) of children who received only one vaccine dose (n = 281) and 37.5% (n = 291) of children vaccinated with at least two doses (n = 776). 4.5% (n = 289) of unvaccinated children in the study received intensive care compared to 4.3% (n = 12) of children who received only one vaccine dose and 5.9% (n = 46) of children who received two or more vaccine doses. Additionally, 1.8% (n = 115) of unvaccinated children required a CPAP compared to 1.4% (n = 4) of children who received only one vaccine dose and 2.45% (n = 19) of children who received 2 or more vaccine doses. The study also finds that a higher proportion of vaccinated children received inpatient care (55%) compared to unvaccinated children (44%).
A retrospective cohort study, published in the Journal of the American Medical Association, (n = 20, 592), conducted in Italy between February 28 and September 4, 2022, found that the non-modRNA NOVAX 2 shot primary series is slightly less abysmal against infection as the modRNA boosters on the market. Estimated VE against infection is 23% after the first dose and 31% after the second dose. However, VE remained much more stable than modRNA shots over the study period dropping from an estimated VE of 41% 29 days post administration to 28% VE after 3–4 months. By comparison the literature on the VE of modRNA boosters shows a rapid decline to negligible single digit VE by this time interval. VE against symptomatic illness hovered around 50% within the same 3–4 month time frame. By comparison, the modRNA booster VE against symptomatic illness usually declines rapidly within that time frame.
A recent blood sample study conducted by Kalkeri et al., which analyzed and compared antibodies from blood samples taken 6 months after patients had received their last Pfizer or Moderna boosters followed by a single dose of Novavax recombinant spike shot (n = 20) with patients who had received only 4 Novavax doses (n = 18) found that recipients of 4 Novavax recombinant spike had >10x higher anti-spike IgG3 (inflammatory) antibodies, which are responsible for 80% of neutralizing activity, compared to recipients of ModRNA boosters while said recipients had >75x anti-spike IgG4 (non-inflammatory) antibodies that are linked to immune tolerance and suppression.
A population based cohort study in Iceland, published in the Journal of the American Medical Association, from December 1, 2021 until February 13, 2022, of PCR positive persons (n = 11,536) found that reinfection rates were highest among the 18–29 year old and 30–49 year old cohorts. Adjusted reinfection rates were also higher among recipients with a complete primary series and/or booster compared to unvaccinated and/or recipients of one dose.
A retrospective population wide study conducted in Austria among previously infected Austrians who had not been vaccinated with non-ModRNA products (n ~4 million) between November 1, 2022 and December 31, 2022, published in The European Journal of Clinical Investigation, found that out of the 69 deaths with COVID that occurred during this interval 31 had received the bivalent booster, 20 had received the original booster only, 11 were unvaccinated and 7 had only received one or two doses of the primary series. There were 8,511 infections among 281,291 recipients bivalent booster recipients (3% of recipients), 37,624 infections among 1.545 million original booster recipients (2.4% of recipients), 22,554 infections among 933,277 recipients of one or two doses of the primary series (2.4% of recipients) and 20,367 infections among 1.225 million unvaccinated persons (1.7% of recipients). From COX proportional hazard ratios, the authors found a relative VE of -24% between the fourth and third dose for COVID mortality and a 17% VE for infections. There were no significant other group differences in COVID-19 mortality, but fewer infections were recorded in the less vaccinated groups (Table 2). A follow up study between January 1, 2023 and June 30, 2023 among recipients of four doses (n = 490,623), three doses (n = 1.352 million), two or one dose (n = 911,896) and the unvaccinated (1.223 million) found that out of 225 deaths with COVID that occurred during this interval 95 had received the bivalent booster, 75 had received the original booster only, 29 were unvaccinated and 26 had only received one or two primary series doses. There were 29,808 new infections among bivalent booster recipients (6.07% of recipients), 80,246 new infections among three dose recipients (5.9% of recipients), 39,156 new infections among primary series recipients (4.3% of recipients), and 24,964 new infections among the unvaccinated (2.04% of recipients). Analyses in 2023 confirm no relative VE for the bivalent booster versus the original booster for COVID-19 mortality (4%, 95% CI: −31 to 29), but show higher risk of infection with a relative VE of −17% (95% CI: −19 to −15) (Table 4). After the follow up study the authors conclude that evidence shows VE peaks at about 3–5 weeks post inoculation but then rapidly declines within a couple of months with ‘no remaining effect beyond 15 weeks was previously reported and fits well to our findings.’ They also found that ‘compared to three vaccine doses, those with fewer or no vaccinations did not differ with regard to COVID-19 mortality but had reduced risk of SARS-CoV-2 infections.’
Not all of matched cohort studies find this inverse relationship but they do find a very low VE that rapidly wanes within a few months. An open label non-randomized clinical study in Israel administered a fourth dose of Moderna or Pfizer 4 months after the third dose to 154 out of 1050 eligible healthcare workers. Each recipient was paired with two aged, matched controls from the pool of eligible healthcare workers. The study found a 25% infection rate in the control group compared to 18.3% of fourth dose recipients (Pfizer) and 20.7% of fourth dose recipients (Moderna) leading to an estimated VE of 30%. researchers ‘observed low vaccine efficacy against infections in health care workers, as well as relatively high viral loads suggesting that those who were infected were infectious. Thus, a fourth vaccination of healthy young health care workers may have only marginal benefits.’
A prior study, published in the New England Journal of Medicine, that extrapolated data from n = 1.25 million vaccine recipients >60 years of age in the Israeli Ministry of Health database, who were eligible for the second booster, found that recipients of the fourth dose initially had lower rates of infection (171 cases per 100K person days), an adjusted rate ratio of 2, compared to the original booster group (340 cases per 100K person days) and an adjusted rate ratio of 1.8, compared to the internal control group (308 cases per 100,000 person days) after four weeks. However, starting at week five and after 8 weeks, the adjusted rate ratio of the fourth dose group declined in comparison to the control group and third dose group to adjusted rate ratios of 1 (i.e. the same rate ratio) for the former and 1.1 for the latter. The authors conclude that: these findings suggest that protection against confirmed infection wanes quickly…The adjusted rate of infection in the eighth week after the fourth dose was very similar to those in the control groups. These findings indicate no (statistically significant) difference.
UK vaccine surveillance data from week 1 of January 2022 found that two doses of Pfizer and Moderna is initially 65–70% effective against symptomatic omicron infection but drops to VE of 10% after 5 months. 2 doses of AstraZeneca has no VE against symptomatic omicron after 5 months. The first booster has an initial VE of 65–70% against omicron but this declines to 55–70% between weeks 5–9 post administration and 40–50% after 10 weeks (2.5 months).
UK vaccine surveillance data from week 3 of January 2022 found that two doses of AstraZeneca was initially 45–50% effective against symptomatic omicron infections but drops to zero after 20 weeks (5 months). Similarly, the primary series of Pfizer and Moderna is initially 65–70% effective against symptomatic omicron but drops to 10% after 20 weeks (5 months). The booster dose for Pfizer and Moderna is also 65–70% effective against symptomatic omicron 2–4 weeks after uptake but was found to drop to 45–50% effective after 10 weeks (2.5 months).
UK vaccine surveillance data for week 23 of June 2023 finds that the monovalent boosters are initially 30% effective against infection, 40% effective against symptomatic disease and 60% effective against hospitalization in the first month. This drops to 20%, 40% and 40% in months 2–3 and 10%, 10% and 20% in months 4–6. 6 months post administration, the monovalent boosters are 0% effective against infection and 0% effective against hospitalization against subvariants BA4, BA5, BQ1 and CH1.1. The bivalent booster does not fair any better producing an initial VE of 30% against infection, 40% against symptomatic disease, and 55% against hospitalization in the first month. This drops to 20%, 40% and 50% in months 2–3 and 10% against infection and symptomatic disease in months 4–6. The bivalent booster VE against hospitalization fairs even worse against XBB1.5 initially starting with a VE of 57% in the first 2–4 weeks but rapidly dropping below 50% in weeks 5–9, dropping to a little more than 25% in weeks 10–14 and dropping to 12% after 15 weeks (3.5 months). The highest number of hospitalization occurred in booster recipients 75+ years of age 3–6 months post administration.
The SIREN Cohort study conducted among vaccinated UK healthcare workers over 18 years of age between September 2021 and February 2022 (n = 19,614) found that the first booster dose had a VE of 63% against infection against the delta variant but this dropped to 32% against infection from the omicron variant within the first two months of its emergence with no additional benefits from the booster after 4 months. Most importantly, the SIREN study found that immunity from a prior infection remained as high as 85% effective against a delta variant infection for 15 months after the primary infection, much higher than the estimated protection odds afforded to recipients of the primary series or booster without a prior infection. With the emergence of omicron this fell to 25% 9 months after the primary infection which while low is still higher than that estimated for the first booster. This study was perhaps one of the first to theorize that mucosal immunity from a prior infection could be a superior route to developing immunity against reinfection and severe disease.
The Expose uncovered evidence of both time and age specific VE in UK Health Security Agency data during the beginning of the Omicron wave from weeks 41–49 of 2021 and week 1 of 2022. Age specific VE against infection drops rapidly from 89% in the Under 18 cohort to 33% in the 18–29 cohort before becoming negative, meaning their probability of contracting COVID is higher, for the older cohorts in week 37–40 of 2021.
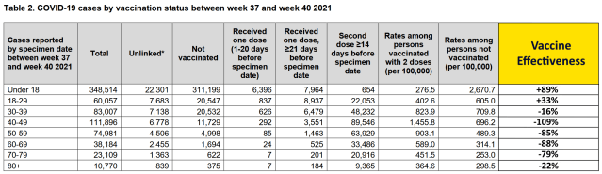
Age specific VE continues to decline rapidly in weeks 41–44 with age specific VE against infection declining to 78% in the under 18 years cohort and 13% in the 18–29 years old cohort and as low as -126% in the 40–49 cohort.
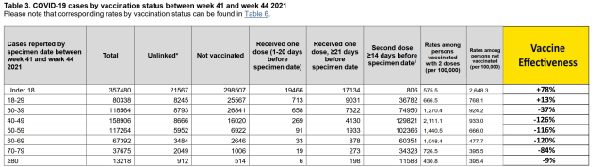
The booster seems to restore partial immunity in the two oldest cohorts between weeks 45–48.
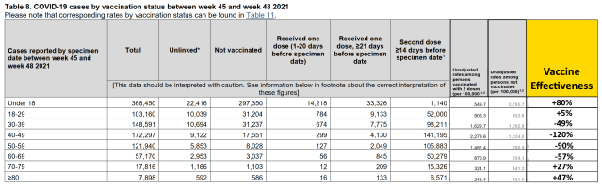
But this is short lived. VE against omicron infection falls to 38% for under 18 years of age and becomes markedly negative for older cohorts.
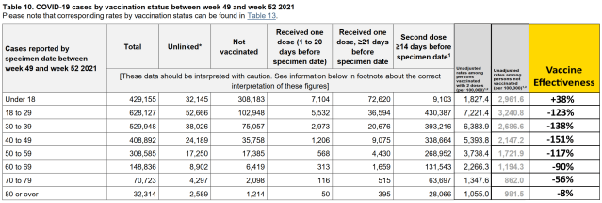
Age specific VE against infection over the entire 5 month period evidences negative immune imprinting in for 1–2 dose recipients for all but one cohort in the beginning of omicron.
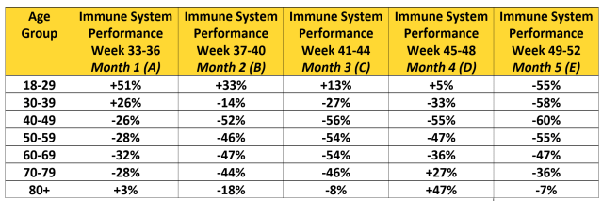
The effectiveness of COVID-19 vaccines fell below 20 percent a few months after vaccination, with booster shots seeing effectiveness drop below 30 percent.
A systematic review published in the JAMA Network journal on May 3, analyzed 40 studies estimating vaccine effectiveness (VE) over time against laboratory-confirmed COVID-19 infection and symptomatic disease. The studies were selected from 799 original articles, 149 reviews published in peer-reviewed journals, and 35 preprints. The review found that the vaccine effectiveness of a primary vaccination cycle against the Omicron infection and symptomatic disease was lower than 20 percent at 6 months from the administration of the last dose.
Booster doses restored vaccine effectiveness to levels similar to those seen after administration of the primacy cycle dose. However, nine months after the booster dose, vaccine effectiveness against Omicron was found to be lower than 30 percent against infection and symptomatic disease.
“The half-life of VE against symptomatic infection was estimated to be 87 days for Omicron compared with 316 days for Delta. Similar waning rates of VE were found for different age segments of the population.” “These findings suggest that the effectiveness of COVID-19 vaccines against laboratory-confirmed Omicron or Delta infection and symptomatic disease rapidly wanes over time after the primary vaccination cycle and booster dose,” the study said. “Putting together the bulk of available evidence on the waning of VE over time against COVID-19 variants has crucial implications for future interventions and vaccination programs.Effectiveness by Vaccine Brand
Vaccine effectiveness against Omicron infection was 44.4 percent a month after the completion of the primary vaccination cycle. This fell to 20.7 percent at six months and then to 13.4 percent at nine months. Vaccine effectiveness was found to be higher against the Delta variant as compared to the Omicron variant.
“Pooled estimates of VE after any primary vaccination cycle against symptomatic disease after Omicron infection show a marked waning over time,” the study stated.
Effectiveness against symptomatic disease fell from 52.8 percent a month after completion of the primary vaccination cycle to 14.3 percent at six months and 8.9 percent at nine months.
“Our estimates suggest that the initial VE could be different depending on the vaccine product, with higher VE found at one month from the second dose administration for Moderna and Pfizer-BioNTech compared with AstraZeneca and Sinovac.”
With regard to age, vaccine effectiveness was found to be similar in younger and older age groups against the Omicron variant infection.
No “significant differences” were observed between the two age groups regarding vaccine effectiveness against Delta variant infection. “A significantly lower VE was found for both age groups for Omicron compared with Delta,” it stated.
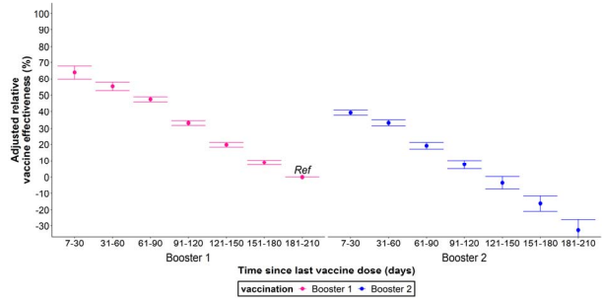
A study of long-term booster effectiveness conducted by Chemaitelly et al., found that the booster is initially 41% effective against infection relative to the primary series but rapidly declined to 14.4% VE in month 6 and became negative thereafter. The booster was found to have a VE of -20.3% against subvariants BA4, BA5 and BA2.75 suggesting negative immune imprinting.
Tseng, 2022 et al., using a test negative case control design to study outcomes for covid patients within the Kaiser Permanente Southern California Health System (n = 4.7 million) found that the first booster VE against BA1 infection dropped from 85%, 14–30 days post vaccination, to 55% after 5 months. Booster VE against BA2 infection fell from 61% to -25% during the same periods. VE against BA.2.12.1 infection dropped from 82.7% to -26.8% over the same time periods. VE against BA4 infection dropped from 72.6% to -16.4% over the same time periods. VE against BA5 infection fell from 90.6% to -18% over the same time periods. The second booster VE against BA2 dropped from 64.3% 14–30 days post vaccination to 17.3% after 3 months. VE against BA.2.12.1 dropped from 64.4% to 14% over the same time periods. VE against BA4 dropped from 75.7% to 6.3% over the same time period. VE against BA5 fell from 30.8% to 5% over the same time period.
“While 3-dose VE against BA.1 infection was high and waned slowly, VE against BA.2, BA.2.12.1, BA.4, and BA.5 infection was initially moderate to high (61.0%-90.6% 14-30 days post third dose) and waned rapidly. The 4-dose VE against infection with BA.2, BA.2.12.1, and BA.4 ranged between 64.3%-75.7%, and was low (30.8%) against BA.5 14-30 days post fourth dose, disappearing beyond 90 days for all subvariants.”
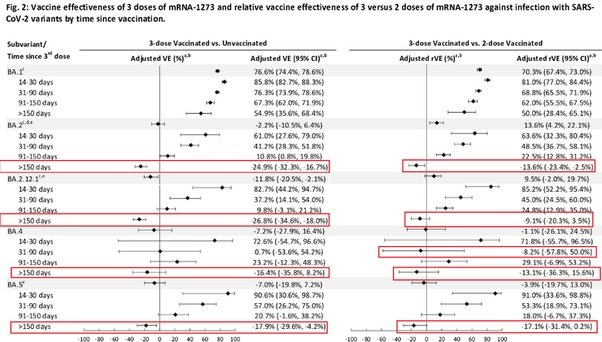
A Danish cohort study reports an initial VE against infection of 55.2% for Pfizer and 36.7% for Moderna after the booster but quickly declines within a few months to 16.1%, 31–60 days after the Pfizer booster, and 30%, 31–60 days after the Moderna booster. Between 61–90 days, the Pfizer booster has a VE of 9.8% while the Moderna booster has a VE of 4.2%. We reach negative VE territory between 91–150 days after the booster.
Tamandjou et al., used a test negative study to estimate VE for the second booster compared to the first booster in symptomatic patients 60 years or older in France between March and October 2022 (n= 933,491). They found that the protection against symptomatic disease offered by the second booster was inferior to the protection offered by the first booster during the same time period after administration with a VE of 39% 7–30 days post administration of the second booster compared to a VE of 64% for the same period after the first booster. The VE of the second booster dropped to 8% after 3–4 months while the VE of the first booster dropped to 33% after the same time period. VE after the second booster fell below zero between months 4 and 5 while VE of the first booster during the same time period did not.
Another matched cohort study examining the efficacy of Molnupiravir accidentally found negative immune imprinting/vaccine dependent enhancement. The study in question is a matched cohort study that was actually examining the clinical benefit of Molnupiravir, within a 30-day window of COVID infection, for previously uninfected veterans at high risk of severe disease. So the study was not meant to directly examine the effectiveness of the primary intervention, the modRNA shot, but it incidentally does this through the results for the control group seen in figure 2.
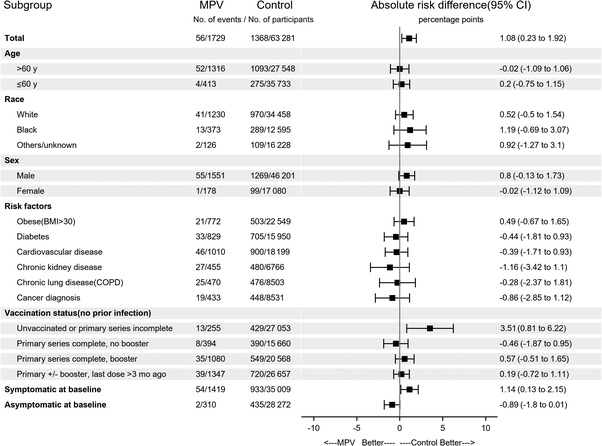
Thus absolute risk breaks down as:
429 hospitalizations and deaths from 27,053 ‘unvaccinated or primary series incomplete’ participants (0–1 shots). (1.6%).
390 hospitalizations and deaths from 15,660 ‘primary series complete, no booster’ participants (2 shots) (2.5%).
549 hospitalizations and deaths from 20,568 ‘primary series complete, booster’ participants (3–4 shots). (2.67%).
720 hospitalizations and deaths from 26,657 'primary series complete +/- booster, last dose 3 months ago.' (2.7%).
This was a matched cohort study, so differences in age and metabolic health were offset.
Another study now published in Nature by Cele et al. notes: Omicron variant escapes antibody neutralization “elicited by the Pfizer BNT162b2 mRNA vaccine in people who were vaccinated only or vaccinated and previously infected.” They reported that Omicron variant “still required the ACE2 receptor to infect but had extensive escape of Pfizer elicited neutralization.”
A Danish cross sectional nationwide questionnaire study (n = 148,874), linked to national testing and registry data and published in Nature Scientific Reports, found that 93.5% of fully vaccinated test-positive respondents reported at least one symptom compared to 92.6% of unvaccinated test-positive respondents with median interquartile range equal to 7 symptoms for the fully vaccinated respondents and 9 for the unvaccinated respondents across three variant reporting periods. During the omicron reporting period fully vaccinated respondents reported symptoms of runny nose, sore throat, cough, shortness of breath, fatigue, and headache more often than unvaccinated respondents. Fully vaccinated respondents reported runny nose 21.3% more often, sore throat 17.6% more often, cough 10.5% more often, shortness of breath 5.9% more often, fatigue 2.7% more often, and headache 2.3% more often.
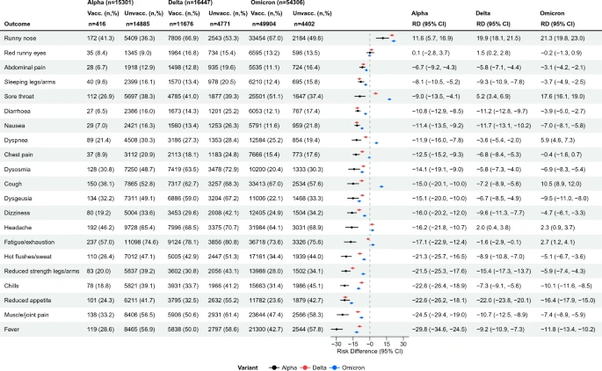
Among test positive unvaccinated respondents, most symptoms were less often reported during omicron compared to delta variant, aside from chills, while the opposite was true for fully vaccinated test positive respondents who reported 11 out of 21 symptoms more often during omicron than the delta variant.
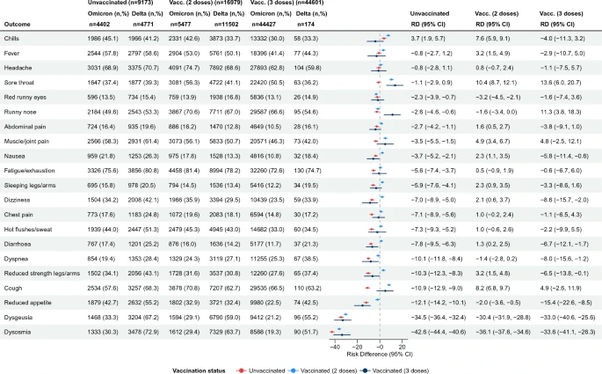
Hoffmann et al., published in Cell also found that: ‘the majority of therapeutic antibodies will be ineffective against the Omicron variant and alarmingly, that double inoculation with BNT162b2 (Pfizer) might not “adequately protect against severe disease induced by this variant.’
A study of Vaccine efficacy among vaccinated children 5–11 years of age within New York (n = 365,502 ) found that VE against infection drops from around 45–55% to about 11% VE within a few weeks. The incidence rate ratio for infection compared to unvaccinated children 5–11 years of age fell from 3.1 on December 13, 2021 to 1.1 (statistical insignificance). by January 24, 2022 (42 days). VE against infection for Children 12–17 years of age dropped from 76% to 46% within the same 42 day time period.
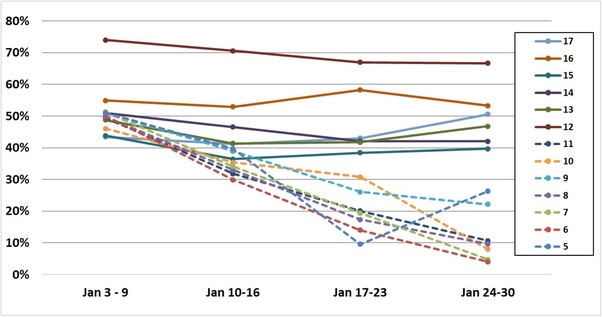
A test-negative, case-control analysis, published in Journal of American Medical Association, observes that the VE of the primary series dropped even faster for children and adolescents for the omicron variant. The analysis examined 43,209 negative test controls and 30,999 positive test cases for children 5–11 years of age (n = 74, 208) as well as 25,471 negative test controls and 22,273 positive test cases for adolescents between 12–15 years of age (n = 47,744) from 6,879 test sites across the U.S. between December 2021 and February 2022. VE against symptomatic omicron infection was estimated at 60.1% for 5–11-year-olds and 59.5% for 12–15-year-olds 2–4 weeks after the second dose of the Pfizer primary series. VE rapidly declined to 28.9% for 5–11-year-olds and 16.6% after 2 months and dropped to almost 0 VE 3 months after the second dose for both cohorts. A different analysis from the same test platform reported VE of 42% against symptomatic omicron infection 2–4 weeks after the second Pfizer dose which dropped to 0 three months after the second Pfizer dose.
Another retrospective cohort study (n =7,292) published in Journal of American Medical Association conducted between January and September 2022, found yet another unremarkable result that the first booster VE is time specific on a very short timeline including for pregnant women and infants. Using an asymmetric 14 day case counting window, they found a marginally lower risk of infection (VE <50%) for infants less than 6 months only if their mother received the booster during pregnancy. The infants of mothers who took the booster before pregnancy had no statistically significant lower risk of infection with mean VE of 15.4% and lower bounds in negative VE territory. Of course, this result does not inspire any confidence in a product with hit or miss efficacy because the fertile women who got the booster won’t always know when they will get pregnant and likely didn’t plan their pregnancies around the booster and probably won’t read this study and plan them around the next booster in the future.
A study in the Journal of the American Medical Association compared neutralization antibodies in blood samples from participants three months after the primary series (n = 73) and the first booster dose 10 weeks (2.5 months) post administration (n = 55). The study found that detectable geometric mean titers against omicron dropped from 76.2% four weeks after the booster, to 53.3% 8 weeks after the booster, to 18.9% 12–14 weeks after administration of the booster suggesting a rapid decline in omicron specific titers after only a few months.
A prospective cohort study of healthcare workers without prior covid infections (n = 11,176) , published in the New England Journal of Medicine, found that the second booster VE against omicron infection declined from 52%, during the first 5 weeks, to -2% at 15–26 weeks (3–6 months). A concurrent study of neutralizing antibodies in boosted healthcare workers (n = 6,113) found that IgG antibody levels peaked at week 4 post administration and gradually declined to baseline levels prior to the second booster.
Wang et al., found that there are no discernible differences in omicron neutralization between the older monovalent (used in the study above) and the new bivalent:
‘Boosting with a new bivalent mRNA vaccine targeting both BA.4/BA.5 and an ancestral SARS-CoV-2 strain did not elicit a discernibly superior virus-neutralizing antibody responses compared boosting with an original monovalent vaccine.’
After analyzing serum collected from individuals who received 3 doses of the monovalent and comparing them to those who received the bivalent (an average of 26 and 24 days after vaccination). All participants exhibited the highest neutralizing titers against the ancestral strain and there were no statistically significant differences in the neutralization of any variant between the groups 3–5 weeks after the booster.
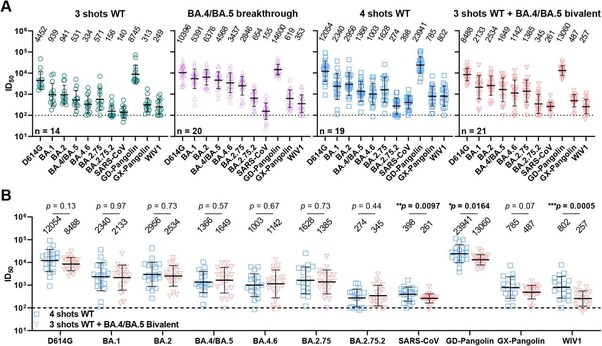
A study published in the New England Journal of Medicine (Davis-Gardner et. al.,) also analyzed serum samples from (n = 35) participants: 7–28 days after the first monovalent booster (n = 11), 6–57 days after the second monovalent booster, and 16–42 days after the bivalent booster (n = 12). The study found substantially lower neutralization activity against all omicron subvariants compared to the wildtype-ancestral variant in all three cohorts. In both monovalent cohorts (n=23)(Geometric Means) antibody titers against BA1 and BA5 were 5–9x lower than against wildtype-ancestral variant and 23-63x lower against BA.2.75.2, BQ1.1 and XBB than the wildtype-ancestral variant. In the bivalent booster cohort, (Geometric Means) antibody titers against BA1 and BA5 were 4x lower than against wildtype-ancestral variant and 12–26x lower against BA.2.75.2, BQ1.1 and XBB than the wildtype-ancestral variant.
Another study published in the New England Journal of Medicine (n = 33) found that both the monovalent (n = 15) and bivalent (n = 18) boosters lead to the expansion of antibody titers for the wildtype-ancestral variant and lower antibody titers for the BA5 omicron variant after a median of 3 doses. Memory T cell responses were barley affected in either group and the bivalent booster produced a slightly higher level of antibody titers at a rate ratio of 1.3.
Another Study published in the New England Journal of Medicine (Hachmann et al.,) confirmed that Pfizer booster produced a much more robust antibody response to wildtype-ancestral variant, with a median 5,783 antibody titers 2 weeks after administration, and a much more subdued response to the omicron subvariants with a median 900 antibody titers against BA1, median 829 antibody titers against BA2, median 410 antibody titers against BA.2.12.1, and a median 275 antibody titers against BA4 and 5 2 weeks after the booster. All but one of the participants infected with BA1 and 2 subvariants were vaccinated suggest substantial immune evasion by omicron subvariants from the booster immunization. Participants with prior infections had median antibody titers of 11,050 against wildtype-ancestral variant, 1,740 against BA1, 1,910 against BA2, 1,150 against BA.2.12.1, and 590 against BA4 and 5.
Another study published in Vaccines, (Sheehan et al.,) collected blood samples from (n = 16) SARS-COV-2 naive booster recipients at 6 times points over 420 days, including prior to vaccination, and found that receptor binding domain and spike-specific IgG4 antibody levels were significantly elevated in boosted but not primary series immune blood samples. IgG1 and IgG3 neutralizing antibody levels peaked at 3 weeks after the second dose and declined after four months. While IgG2 and IgG4 levels were initially negligible during the primary series the booster dose induced changes in the subclass distribution. While receptor binding domain and s-protein reactive IgG2 and IgG3 were not detected 6 months after the booster antigen specific IgG1 and IgG4 antibodies persisted after this duration. Neutralizing titers declined to pre-immune levels 6 months after the booster.
While boosters enhanced serum IgG Ab reactivity and nAb responses against variant strains, all variants tested showed resistance to two- and three-dose immune sera. Our data reflect the poor durability of vaccine-induced nAb responses which are a strong predictor of protection from symptomatic SARS-CoV-2 infection. The induction of IgG4-switched humoral responses may permit extended viral persistence via the downregulation of Fc-mediated effector functions.
A rodent study published in iScience additionally finds that repeated boosters that target the receptor binding domain impairs serum neutralization, the activation of T memory cells, and leads to immune tolerance. Immune tolerance is the body’s inability to produce antigen specific antibodies that activate memory T cells or insufficient production of them upon encountering a new infection. The study detected a 2.5–4x reduction in average antibody titers against the delta and omicron variants compared to the wild type and a (statistically) significant reduction in the serum neutralization in the extended (boosted) group against all 3 types compared the the conventional (primary series) group.
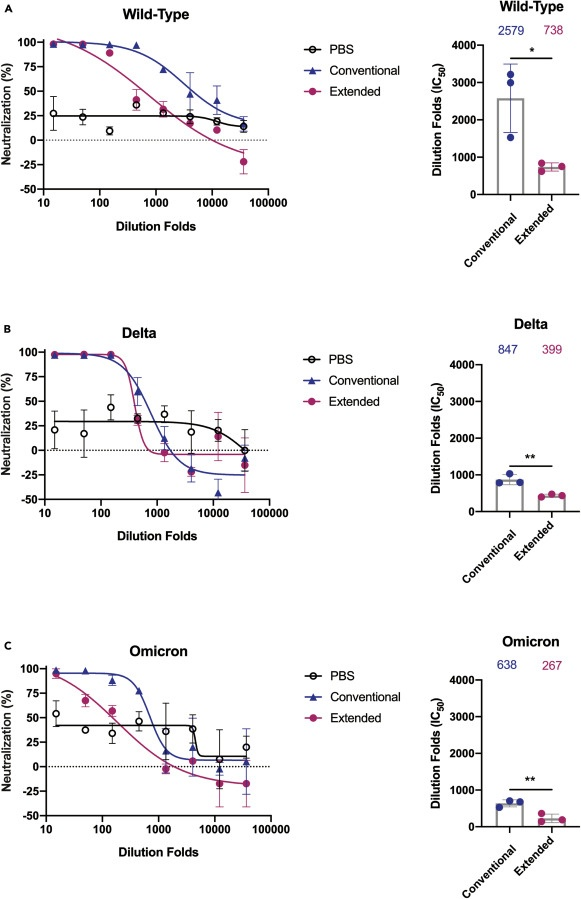 Note: PBS is the control group
Note: PBS is the control group
A study published in Nature Medicine Kurhade et al., tested the neutralizing activities of both of the two monovalent and one bivalent in 3 serum panels collected from participants 23–94 days after their fourth dose between March and October 2022 (n = 77). The three panels consisted of 1) n=25 blood samples from individuals 23 - 94 days after the 4th monovalent 2) n = 29 blood samples from individuals 14- 32 days after the bivalent and 3) n = 23 blood samples from individuals with previous infections and received a bivalent 14–32 days ago. The study found that geometric means titers against BA4&5 (298), BF7 (305), BA4.6 (183), BA2.75.2 (98), BQ1.1 (73) and XBB1 (35) were 12.1x, 11.9x, 19.8x, 36.9x, 49.6x and 103x lower than the geometric mean titers against wildtype-ancestral variant (3,620) 14–32 days after the bivalent. The study found geometric mean titers of against BA4&5 (95), BF7 (69), BA4.6 (62), BA2.75.2 (26), BQ1.1 (22) and XBB1 (15) were 16.1x, 22.2x, 24.7x, 59x, 69.7x, and 102x lower than the geometric mean titers against wildtype-ancestral variant (1,533). The study found that infected blood samples had the highest levels of neutralizing antibodies against all omicron subvariants and the wildtype-ancestral variant (5,776) with geometric mean titers of BA4&5 (1,558), BF7 (1,223), BA4.6 (744), BA2.75.2 (367), BQ1.1 (267) and XBB1 (103). The authors conclude: 1) immune evasion has increased with new omicron subvariants and 2) infection confers higher and broader neutralization against circulating subvariants.
A recent antibody study published in The Lancet analyzed neutralization activity against newly emerging subvariant BA2.86 in blood samples taken from monovalent and bivalent boosted participants found that BA.26 evades booster induced humoral immunity. The three monoclonal antibodies that worked against the parental BA2 parental subvariant had no effect against BA.26 infections have been detected in 11 countries and the subvariant’s spike protein is 30 mutations separated from the parental BA2 subvariant. BA2.86 reproductive rate is 1.3x higher than XBB1.5 and similar to the reproductive rate of EG5.
A Canadian seroprevalence study funded by their Covid Immunity Task Force found that only 9% of Canadians had acquired immunity to COVID through infection by November 2021, when 75% of Canadians had completed the primary series and 80% had taken at least one shot. This rapidly increased with to 76% of Canadians in March 2023, regardless of how many injections they had taken. The synopsis notes that most Canadians were infected during the omicron wave after previous vaccination. In 2022, deaths attributed to COVID19 increased 36.6% from the prior year, from 14,466 to 19,716, including the proportion of deaths among senior citizens (91.4%), despite achieving a vaccination rate of >80% in 2021. Life expectancy also fell for the third consecutive year.
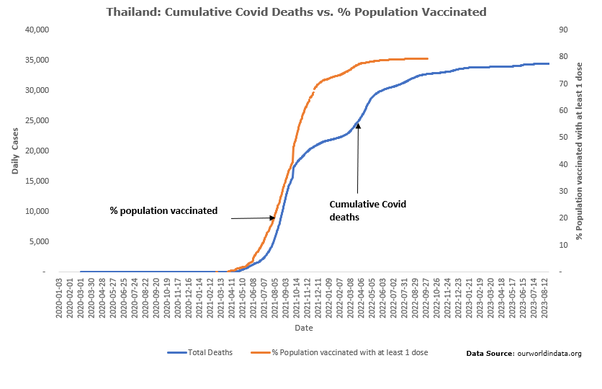
Thailand recorded 80 deaths from 24,400 confirmed covid infections (CFR = 0.327) 11 months after their first confirmed COVID case on Jan 8, 2020. 11 months after their vaccination rollout on Feb 14, 2021, Thailand recorded 20,445 deaths from 2.253 million confirmed cases (CFR = 0.9)
In Australia, a follow up survey was conducted with 11,697 participants who were recently diagnosed with an omicron infection. 18.2% of them (2,130) met the criteria for long COVID 90 days post diagnosis of their first omicron infection. Females were found to have a 50% higher risk of developing long covid compared to males and individuals with comorbidities had a 50% higher health risk compared to individuals with no pre-existing conditions as well as older adults compared to younger adults. 94% of participants had received 3 or more shots. The boosters were estimated to reduce the risk of long covid by 40% (RRR) though most of the variance in outcome is not explained by the booster but metabolic health.
A population based survey of (n=17,008) people in Germany with prior infections, published in the International Journal of Infectious Disease, found that 70% of participants were vaccinated at the time of infection and 50% had received at least 1 booster shot, with 68% of infections occurring during omicron. About 8% of participants experienced more than one infection. 2,822 reported persistent symptoms 12 weeks or more after infection and met the criteria for long COVID. The vast majority are female, 2081 participants, over the age of 40 years, 2,054 participants, and experienced moderate to severe symptoms, 1,906 participants. The highest risk for Long Covid symptoms was associated with a wild-type ancestral variant infection with >50% cases leading to the development of long COVID symptoms. This gradually declined with the emergence of Alpha (47.2%) and Delta (26.13%) variants and rapidly declined with the emergence of omicron (11.36%) for unvaccinated individuals with no prior infection. The risk of long COVID also gradually declined for vaccinated individuals who received 1–2 doses of the primary series between the Alpha variant (42.14%), Delta variant (27.12%) and omicron (16.76%). Booster recipients had a lower risk of long COVID after any Delta infection (23%) but a higher risk of long COVID after any omicron infection (13.17%) compared to unvaccinated individuals. Based on adjusted odds ratios, the results show that vaccination conferred no meaningful protection against long COVID after infection but provides evidence that a prior infection reduces the risk of long COVID. The study also found that among those who developed long COVID symptom severity was similar across all variants and did not differ by vaccination status or prior infection. The most commonly reported symptoms of long COVID are fatigue (76.1%), shortness of breath (59.6%), and cognitive impairment (59.4%).
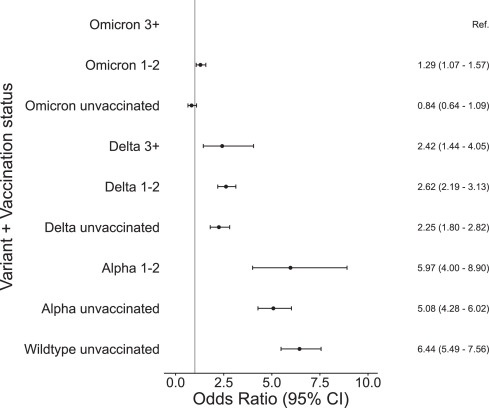
A systematic review of VE against long COVID symptoms, published in Cambridge University Press, evaluated 32 studies (n = 775,931), 24 of which occurred prior to the omicron variant, and found that VE against long COVID, defined as persistent COVID19 symptoms for 4–26 weeks, was approximately 30% for recipients who received at least two primary series doses prior to infection, but that the vaccine conferred no protection against long COVID if infection occurred prior to injection. In fact, the pooled odds ratio for recipients who were infected prior to being fully vaccinated of developing long COVID, OR=1.3, suggests such vaccine recipients are slightly more likely to develop long COVID symptoms than the unvaccinated. 7 studies that evaluated long COVID symptoms for recipients who were vaccinated prior to any omicron infection (n = 155,710) found a VE of 31.6%, roughly the same as the other 25 studies in this systematic review.
A community-based test negative study conducted with vaccination and covid19 infection data from patients 65 years of age or older from 3 Japanese municipalities (n = 20,000), and published in BioMed Central Journal, found a mean VE of 33.6% for two or more monovalent and one bivalent shot compared to no shots 35 days after administration of the bivalent or last monovalent shot and a relative mean VE of 18.2% for the recipients of the bivalent shot compared to patients who had stopped with the primary series within a 35 day post administration window. While these results look positive, albeit marginal, for the boosters and primary series the study found one big caveat: there was no statistically significant VE for seniors with prior covid infections. As the authors note: Furthermore, although bivalent vaccines were effective among those without a history of infection, we did not find sufficient evidence of the effectiveness of bivalent vaccines among previously infected older adults.