Science Experiment : Simple Model Of Fire Extinguisher
Hello all steemians and science lover. Now i want to share about how to make Simple Model Of Fire Extinguisher This experiment aims how firefighters tools work.

Basic Theory
A fire extinguisher is an active fire protection device used to extinguish or control small fires, often in emergency situations. It is not intended for use on an out-of-control fire, such as one which has reached the ceiling, endangers the user (i.e., no escape route, smoke, explosion hazard, etc.), or otherwise requires the expertise of a fire department. Typically, a fire extinguisher consists of a hand-held cylindrical pressure vessel containing an agent which can be discharged to extinguish a fire. Fire extinguishers manufactured with non-cylindrical pressure vessels also exist, but are less common.
There are two main types of fire extinguishers: stored-pressure and cartridge-operated. In stored pressure units, the expellant is stored in the same chamber as the firefighting agent itself. Depending on the agent used, different propellants are used. With dry chemical extinguishers, nitrogen is typically used; water and foam extinguishers typically use air. Stored pressure fire extinguishers are the most common type.
Cartridge-operated extinguishers contain the expellant gas in a separate cartridge that is punctured prior to discharge, exposing the propellant to the extinguishing agent. This type is not as common, used primarily in areas such as industrial facilities, where they receive higher-than-average use. They have the advantage of simple and prompt recharge, allowing an operator to discharge the extinguisher, recharge it, and return to the fire in a reasonable amount of time. Unlike stored pressure types, these extinguishers use compressed carbon dioxide instead of nitrogen, although nitrogen cartridges are used on low temperature (-60 rated) models. Cartridge operated extinguishers are available in dry chemical and dry powder types in the U.S. and in water, wetting agent, foam, dry chemical (classes ABC and B.C.), and dry powder (class D) types in the rest of the world.
Materials and tools
- Vinegar, 25 ml
- baking soda, 50 grams
- Candles, 1 piece
- Glass, 1 piece
- Matches, 1 piece
- Spoon, 1 piece
Ways of Working
- Place the candle in a glass like the picture.
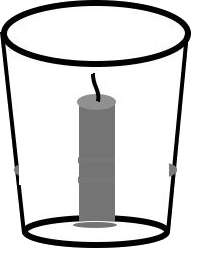
- Sprinkle baking soda.
- Light the candle.
- Pour the vinegar into the glass.
- Observe what happens?
- Conclude the benefits of experiments in everyday life
Conclusion
This medium is used in experimental activities to show the reaction between baking soda and vinegar. The reaction between baking soda and vinegar can be utilized for human life. In this medium, the experiment produces a reaction between vinegar and baking soda to produce sodium acetate and carbonic acid. The resulting carbonic acid is readily decomposed into water and charcoal gas which can extinguish the flame. The CO2 produced in this experiment is around the candle, so the oxygen-deficient candle causes the fire to wax out.
Source :
What does Fire Extinguisher mean?

This post recieved an upvote from minnowpond. If you would like to recieve upvotes from minnowpond on all your posts, simply FOLLOW @minnowpond
This post recieved an upvote from minnowpond. If you would like to recieve upvotes from minnowpond on all your posts, simply FOLLOW @minnowpond