Chemistry 101: Why does pressure increase when you increase the temperature?
The common answer is because the molecules have a higher kinetic energy. That is correct but that is just a statement with no justification. How can we justify that?
First we must define some common equations:
Next we need a system to apply these equations to:
The image shows a particle hitting a wall, what it does not show is the molecule bouncing back. In classical mechanics we can show total momentum as sum of the particle going towards the wall and bouncing back.
More Equations
Volume is typically written is Meters cubed. When we apply both formulas as shown below it gives the volume in units of meters cubed so it shows that the calculation is correct. Next the density and number of particles is defined. Na is Avogadro's number which its units are particles per mole. This cancels out the moles if we did the denominational analysis which leaving only the number of particles.
The reasoning for defining these equation is solve for the total momentum of the system. We then must multiply by all the particles that we have so the equation that follows is:
Since we only want the pressure exerted on the wall then we must divide by 2 followed some simplification
ANNNNNNND there is more
I just realized I made a mistake with t it should be Delta t and momentum should be change in momentum. First F is found and then it becomes substituted into the pressure formula.
In order to get the mean velocity in all 3 dimensions some vector calculus is used. However there is only the need for one third since we added two extra dimensions.
The formula below was determined empiracally through various experiment and it holds true for ideal gases. If you've made it this far into the post I will assume you know what I'm talking about.
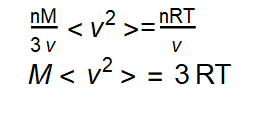
Finally dividing both sides by 2 to yields:
Somebody had to do it... Now when people search for this problem it will be here on steemit. I could not find a sensible justification somewhere so I made my own. This will probably be one of top 5 least appreciated posts since the percent of the audience who can understand this so low. Whoever was able to follow kudos haha. Back to making 2 cents for 3 hours of work.
Congratulations @vexedkiller007! You have received a personal award!
Click on the badge to view your Board of Honor.
Do not miss the last announcement from @steemitboard!
Congratulations @vexedkiller007! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!