Thermodynamics Review Problems for Mechanical Engineering Students | Series 25
This is the 25th series of my "Thermodynamics Review Problems for Mechanical Engineering Students". If you've missed the previous series you may try scrolling this blog and head over to the "Curriculum". This series features a review problem for Three-process Thermodynamic cycles for which two months ago I made two blogs about this matter. See, Part 1 and Part 2.
Review Problem
A three-process cycle operating with
5 kgm of air as the working substance has the following processes: constant volume (1 -> 2); constant pressure (2 -> 3); and constant temperature (3 -> 1). Given that P1 = 100 kPa, T1 = 300°K and V1 / V3 = 6, determine the heat added in kJ.Solution
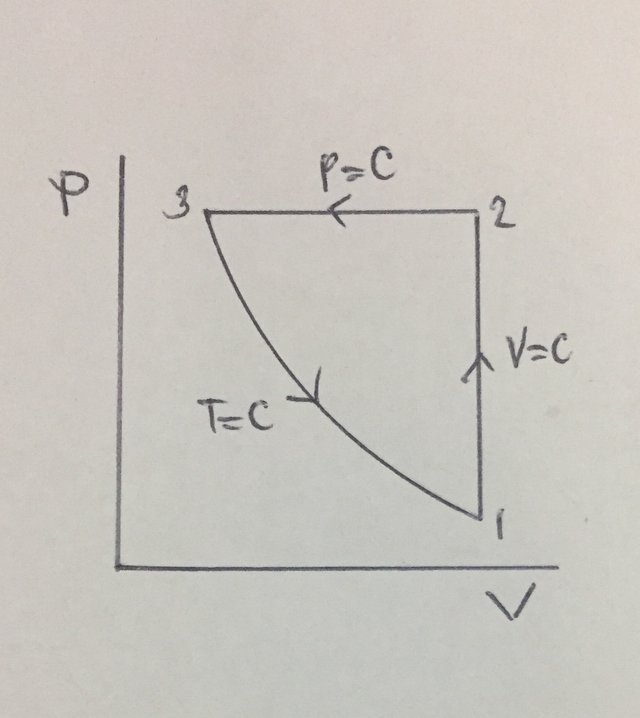
For this review problem, the very first thing to do is to **draw the P-V diagram** of the said three-process cycle. After creating the P-V diagram of the said cycle, by inspection we can say that the heat that is being added to the cycle is done during the isometric process which is literally process 1 -> 2 and is the only one providing a positive sign heat which means heat that is being added. As we proceed to the computation, the first thing to obtain is the pressure at the start of isothermal process which is
P3, and it can only be obtain using the pressure and volume relations during an isothermal process. By using the provided ratio for volumes V1 and V3, we’ve found out that the P3 is equal to 600 kPa. Additionally, since process 2 -> 3 is an isobaric one or constant pressure, P2 is also equal to 600 kPa. Next thing to obtain is the temperature at the end of isometric process, for which we can obtain it using the pressure and temperature ratio during a constant volume process. By using the relation, we’ve found out that 1800°K. And since we now have both T1 and T2, we can now finally solve for the heat that is being added to the cycle and we’ve found out that it is equal to 5389.5 kJ.P-V |
 |
P3 |
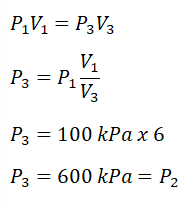 |
T2 |
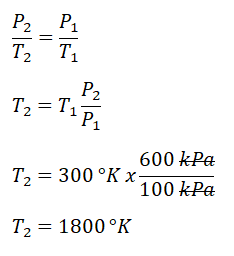 |
QA |
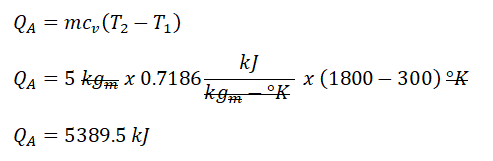 |

Curriculum

Reference
- PIPE - PROBLEM SET #3 by Alcorcon Engineering Review Center
- Sta. Maria, H. [1990] 2012. Thermodynamics 1. Mandaluyong City: National Bookstore Inc..


Congratulations! This post has been upvoted from the communal account, @minnowsupport, by josephace135 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.