The Universe was Born and Destined for Chaos - The Biography of Energy
Introduction
This article is about the incredible story of how we discovered the rules that operate the universe. It's a story of how we found out that all different kinds of energy are destined only to diminish and fall apart, to progress from order to disorder. It's a story of how the universe has followed these laws and created everything that we see around us today.
looking at the picture to the right, one may wonder how we humans managed to change the world like this. We have come a long way in our abilities to capture and control energy, using it for our survival because we now know how important it is to our fragile life. Energy is important to every one of us, we use it to protect ourselves, we use it for transportation, we use it for electricity to maintain the brightness at night. Our body needs it to stay alive and thus, gets energy from the food we eat. But what exactly is energy and why is it so important to life?
Attempting to answer this mind-boggling questions, different scientists will discover weird laws strictly followed by the universe. This discoveries at different places and times will end up connecting our understanding of the chain between stars, humans, and engines. It turns out that apart from being just important for our lives, energy also allows us to make sense of the universe.
Order and disorder
During the late 1600s, a Physicist and Philosopher, Gottfried Leibniz, decided to perform and study experiments that would help answer this question about energy. He was convinced that a universe is a large machine that was designed by a wise person, in experiments he would wonder, "What happens when objects collide?" Observing two balls hit each other he noticed that it was as if something was being exchanged between them during contact with each other. He called whatever was being exchanged - The “Living-Force” and thought of it as a tangible substance that gets exchanged during collisions.
He figured that if men could control this living force, it would change lives and so he became obsessed with this creed. He realized that the life force of an object can be released in all of a sudden! Like an explosion of heat, and heat could be converted into work. About 150 years later, the British Society had completely taken advantage of this discovery and used it to create powerful steam engines for themselves improving their economy forever. But no one completely understood what they were doing, YET!
Thermodynamics
A French scientist called, Nicolas Leonard Sadi Carnot, was driven by circumstances to start his study of heat and motion, and in the process, he developed a new science called Thermodynamics. He was in the military and had his dedication to his country. During a clash with the English people, he and his team were forced to retreat due to heavy firearm owned by the opposing side. The English men had long taken advantage on energy but they still didn't know what it was. The humiliation caused Nicolas to swear that he would find out how these steam machines work and utilize his science for his country to take revenge.
During his studies, he discovered that all heat engines go through a process of heat transfer from hot to cold between objects. He reasoned that heat must be some kind of substance like water that can flow to another body. Then it followed that just like water falling from a top, the flow of heat could be tapped and used to cause motion or in other words 'work (W)'. He figured that to get an engine to use more energy for work, one would have to increase the temperature and the flow of heat will increase respectively. He explained that they were tapping into the flow of the 'living force', in another form (heat), as it moves from one place to another.
This single knowledge has aided engineers for more than 200 years. Jet engines are more efficient than car engines only because the jet engines have been built in such a way that they can hold and use incredible temperatures better than cars. Later, Carnot's love for his country drove him to study the spread of a disease that spread in France. He gave himself so much to finding its cure and saving his home until unfortunately he caught the disease and died the next day, he was just 36.
By the 19th century, scientists and engineers have already done numerous studies on energy and discovered quite a lot. They now understood how different types of energy relate to each other, they now knew the quantity of energy needed to produce another quantity of a different kind of energy. "The amount of energy needed to increase the temperature of 30ml of water by 1 degree Celsius is equal to the amount needed to lift a 12.5 kg object by one meter". What this implies, and people should understand is that: "just as heat, gravity, light, etc. are different forms of energy - even if heat and mechanical work may appear very different on the surface - they are both just two distinct sides of the same thing! The living force as Leibniz called it, or 'Energy' as we call it". This idea is what will then come to be known as The FIRST LAW OF THERMODYNAMICS.
Making it clear to us that energy cannot be created or destroyed in a closed system, but it can be transformed from one form to another. This meant that the total amount of energy in the universe is incidentally fixed! The Universe is one big system that changes energy into many different forms. So in a car, energy is not generated it just changes heat to motion. But this pushes into our mind some dissatisfaction. Like, what is actually happening when one form of energy changes into another type of energy? In fact, why does it do it at all? The answer to this question was driven to us by the studies of a German scientist in the mid-1800s named Rudolf Clausius.
The Second Law of Thermodynamics
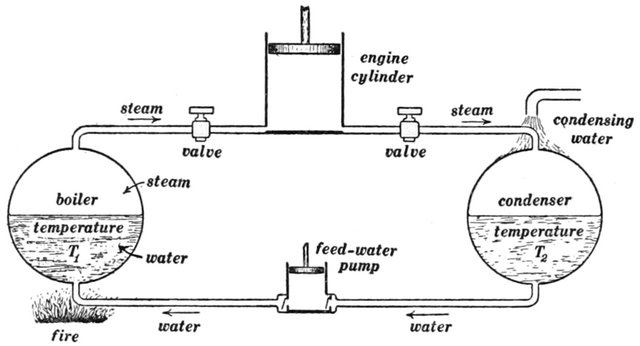
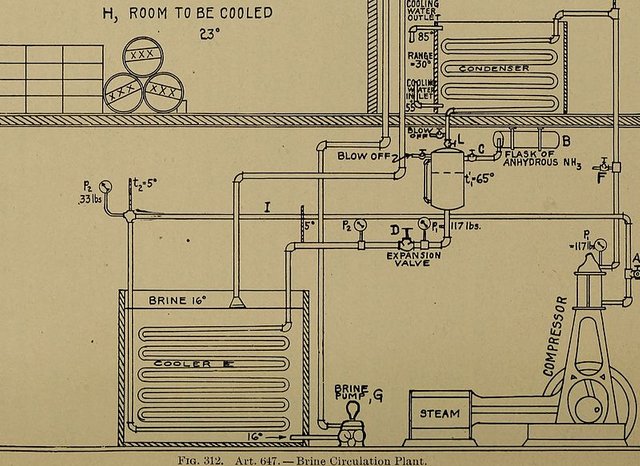
Image - Wikimedia Commons (CC0)
diagram for fixing in the reader's mind the various temperatures and quantities of heat and work which are involved in the operation of an engine
Rudolf Clausius was the first man to present a complete mathematical description of how thermodynamics worked. Carefully observing, he realized that not only does the universe have a fixed amount of energy but that this energy seems to be strictly following a distinct rule! "Heat (if left alone) cannot move from a colder body to a hotter one". This hot cup of tea on my desk as I type will always cool down. This is the only part and direction it follows, energy in form of heat in the cup will flow from the tea through the cup to the table and continue to flow out to the environment until everything is at thermal equilibrium, i.e. Everything around it is equally hot or cold. The flow of heat is a one-way process that has been built deeply into the character of the universe.
It is true that objects can get hotter, but you always need to do something to the object. There has to be an external work done for any object to get hotter or increase its internal energy, left alone, energy has only one fate; from being concentrated to being diminished. Gathering all his knowledge on energy, Clausius introduced a new quantity called entropy and accepted that as heat flows from hotter to colder bodies, entropy also increases simultaneously. He said that entropy is a measure of how heat spreads out and in any closed system, this process is irreversible.
EQUATION [dS/dT = & > 0]
The second law of Thermodynamics where 'S' = entropy and 'T' = time.
Clausius reasoned that this phenomenon also happens everywhere in the larger frame of the universe and that the entropy of the world is accelerating to a maximum, there is nothing we can do about it.
Summary
Everything that allowed the flow of heat is part of an irreversible process that is taking place everywhere in the universe, it is a process of spreading, of dispersing, its a process of increasing entropy. now it feels like our universe shares the same fate as my hot cup of tea.
Join me in the second part of our journey on energy in the next article. We will see why although the science of thermodynamics was successful in the 19th century there was still a very great controversy about the subject. what exactly was this weird thing called entropy? why does it only always increase, changing from order to disorder? Answering this question will lead us to understand how the universe really works, in random chaos.
References
Preposterous Universe - Universe out of Chaos
Book - University Physics 14th ed by Hugh D. Young Roger A. Freedman University of California, Santa Barbar

Hello @agbona
The title is quite scaring I must confess. I almost thought the world is coming to an end 😂, but reading the article and understanding the real gist of the article made me to appreciate the title was a kind figurative expression — though extreme.
The article is a good representation of history (indeed biography) of energy.
Regards
@eurogee of @euronation and @steemstem communities
The truth is, the world is coming to an end, but not really soon...
Thanks for stopping by as always..see you around!
This is sad
I couldn’t agree less
This is an interesting piece
Thanks for sharing
Many thanks @florae always a pleasure having you around here
Congratulations @agbona! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on the badge to view your Board of Honor.
If you no longer want to receive notifications, reply to this comment with the word
STOPHi @agbona!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
You buttressed the point of the title of your article with precise information.. I almost thought we would hear a story about the end of the world.
Great stuff @agbona
Well, I am heading to the story about the end of the world, this is just the part 1 of a Biography of Energy series. I have previously written about the 3 scenarios of how the Universe (world) will end
Thanks for the feedback
Haha.
The science of apocalypse -- That's all I could see here @agbona
Will be on the lookout for part two.
Hey buddy, the part two is out Entropy - The Biography of Energy