OZONE, THE EARTH'S UMBRELLA : ITS DEPLETION.
Ozone is a blue gas that is made up of three oxygen atoms and is created during the photolysis of oxygen molecules into individual atoms by ultraviolent sun rays. These individual atoms further react with atomic oxygen to form ozone. 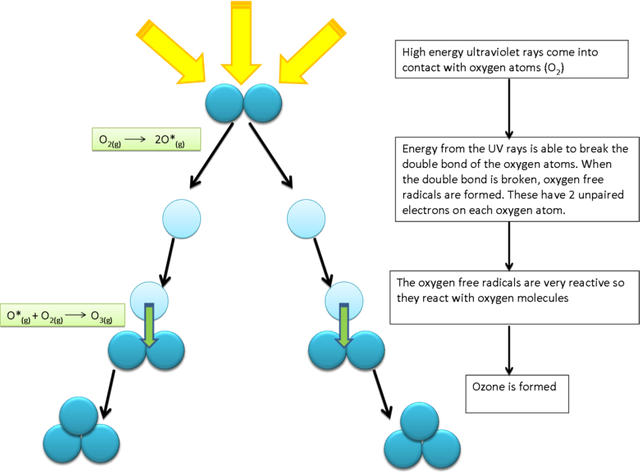
The ultraviolent sun rays that are responsible for the breakdown of molecular oxygen are known as uv-c radiation. Since the reactions for ozone formation have to take place in the presence of continuous ultraviolent radiation, the tropics are known to produce the largest amount of ozone.
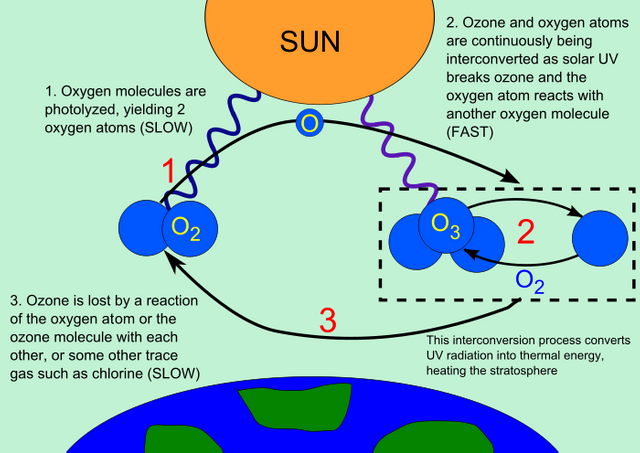
Ozone is mostly concentrated in the stratosphere (10-50km above earth's surface ) and it is in the tropical stratosphere that most of the world's ozone is formed before it is spread all over the rest of the world by stratospheric winds. However, ozone can also be found in the troposphere (0-8km above the earth's surface), although this is an indication of air pollution caused by the burning of fossil fuels. Tropospheric ozone is poisonous and does not contribute to the effects of stratospheric ozone. Stratospheric ozone forms an umbrella-like mass above the earth that functions essentially in preventing ultraviolent rays from reaching the earth by screening out the harsh rays called uv-b radiation. When ozone absorbs uv-b radiation, it naturally gets destroyed in the stratosphere by being broken down into an oxygen molecule and atomic oxygen. 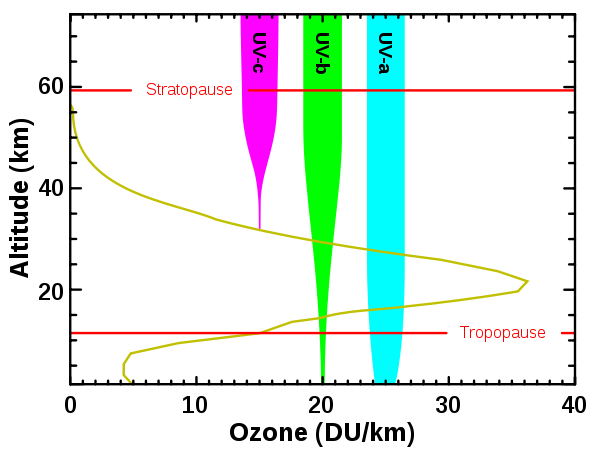
Although the atomic oxygen combines with an oxygen molecule, and the ozone is reformed.
Ozone depletion began with the use of ChloroFluoroCarbons, even though the awareness was not until later. ChloroFluoroCarbons are noble gases that were mostly used for refrigeration, propellants and other industrial purposes for which they were favourable because they did not interfere with the other ingredients of production. 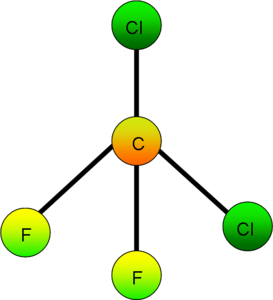
ChloroFluoroCarbons were however discovered to not react in the troposphere, they were noticed to rise to the stratosphere over time where they can be dissociated by ultraviolent rays. The products of these dissociations include chlorine atoms tgat are very reactive. These chlorine atoms react with ozone catalytically to produce chloromonoxide and an oxygen molecule. Chloromonoxide is a radical and is very reactive so it reacts with free atomic oxygen, yielding a free chlorine atom that can catalyse the dissociation of another molecule of ozone.
At this rate, one atom if chlorine can destroy thousands of ozone molecules before it gets exhausted or transported back to the troposphere, where it usually leaves the atmosphere add HCl.
Halomonoxides (monoxides formed with members of the halogen group) are generally catalysts in the dissociation process of stratospheric ozone. Bromine which is used in fire extinguishers in the form of halons ,tends to dissociate stratospheric ozone faster than chlorine. Even if the use of all ChloroFluoroCarbons stopped today, the depletion of atmospheric ozone would still continue until about a hundred years later because the rate of transition of these CFCs and halons from the troposphere to the stratosphere is about 80 years and 65 years respectively.
The effects of ozone depletion are too numerous today but the uv-c radiation which the ozone helps to prevent can destroy DNA, RNA and other macro molecules. Some of the major problems imposed by this global issue include: increment in the cases of skin cancer, plants would be destroyed,marine organisms would be greatly affected and there would be a general risk to life on earth.
NOTE THAT : Fluorine is not effective in the process of stratospheric ozone depletion.
One of the major ways to control the depletion of stratospheric ozone is the ban of industrial and domestic appliances and compounds that depend on the CFCs and halons. This method of approach has proven to be not entirely applicable as the appliances and compounds are still illegally smuggled into some parts of the world especially Africa; although stricter laws and border checks would help. Another method of controlling this global situation is the capture of pollutant emissions instead of their direct introduction into the atmosphere.
I hope you enjoyed reading.
REFERENCES
See here