The Chemistry of Sulphur
The Chemistry of Sulphur:

Cumbustion Of Sulphur
Many people around the world are unaware as to the importance of sulphur in our everyday lives. We go on with our day, unbothered by the everchanging world we are living in. We do not star gaze or question our existence anymore. Have you ever wondered why we live the way we do and how we, the most developed species on Earth, survive? The simpliest answer lies in the constitution of the air we breathe and bodies we inhabit, Sulphur.
Sulphur is one of the most widely used element in the world due to its large range of uses including domestic and commercial uses and is absoluely vital in the existence of man. It has a natural appearance of a yellow crystalline solid and has the chemical and physical properties of being a multi-valent nonmetal, tasteless, odorless and found in nature. The crystallography of Sulphur, which is the arrangement of atoms in a crystalline solid, is extremely intricate and complex. Dependent on induced conditions within a laboratory or in nature, Sulphur allotropes (which are modification to an elements structure, whereby the atoms are bonded together in a different manner) form many unique crystal structures.
The following properties apply to Sulphur on a microscopic level:
- Atomic number: 16
- Atomic mass: 32.06 g/mol
- Electronegativity according to Pauling: 2.5
- Density: 2.07 g/cm^3 at 20 °C
- Boiling point: 445 °C
- Melting point: 113 °C
- Van der Waals radius: 0.127 nm or 0.127*(10^-9) m
- Ionic radius: (+6)
- Isotopes: 5
- Electronic shell: [Ne] 3s(^2) 3p(^4)
- Energy of first ionisation: 999.3 kJ/mol
- Energy of second ionisation: 2252 kJ/mol
- Energy of third ionisation: 3357 kJ/mol
- Standard potential: - 0.51 V
Effects of Sulphur on the Body
Many people may know CHON (carbon, hydrogen, oxygen and nitrogen) as they are deemed the most important elements for a human body as its constituent’s majority of all organic material, but what people may not know is that Sulphur deserves the same level of recognition as it is equally as important. All living organisms require Sulphur for survival and it has an increased importance for humans as Sulphur forms part of methionine which is an amino acid that plays a vital role in our dietary needs. Another important amino acid required for body growth is cysteine. On average, it is recorded that the average human consumed almost 900 mg of Sulphur in the form of protein daily.
Elementary Sulphur does not negatively affect the body as it is not considered toxic towards the body, but when chemically bonded with other elements to create new molecules, could become toxic to the body. Examples of harmful derivatives of combination Sulphur is Sulphur dioxide and hydrogen sulphide. The following repercussions upon ingestion or contact with certain harmful Sulphur compounds may occur:
- Neurological damage
- Behavioural changes and mood swings
- Cardiac damage
- Optical obstructions
- Failure of reproductive organs
- Immune system damage
- Digestive system failure
- Damage to renal functions and liver.
- Upsets in hormonal metabolism
- Hearing damage
- Epidermis issues (dermatological problems)
Sulphur is found in large underground deposits and is extracted from the ground by a process known as the Frasch Process, which was created by a German Chemical Engineer called Herman Frasch.
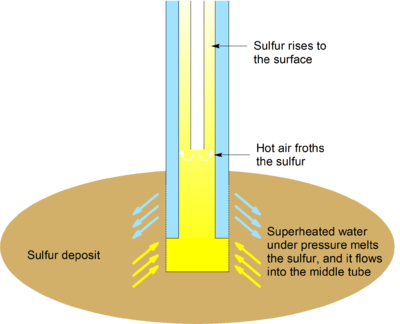
The Frasch Process
Water experiencing extremely high pressures and temperatures (referred to as superheated water) is pumped underground in the outermost pipe to cause the sulphur to melt. In the innermost pipe, compressed air is pumped down. This air mixes with the now liquid sulphur and forms an emulsion that is amazingly less dense than water. Due to the it being the lighter fluid in the deposit, it rises to the surface and is forced up the centre pipe. The purity of this Sulphur is almost 100% (approximately 99.5%)
Applications
As renowned for its domestic use in everyday homes, Sulphur is mainly known for its production of Sulphuric Acid (H2SO4) also known as The Contact Process, which is used in industry worldwide, which will be discussed later in detail. Commercial products due to the manipulation of Sulphur includes cleaning detergents, fertilizers, weaponry such as gun powder and even fireworks and explosives. For more specialized applications of Sulphur, an example of corrosion resistant concrete is used. This type of concrete has a high resistance to frost and therefore can be used in extremely unfavorable conditions, and also has greater strength than that of normal concrete found along street sides and buildings. Specialized additional uses of Sulphur are also inclusive of chemical industries such as pharmaceuticals and also in solvents in a laboratory.
Sources of Sulphur

Sulphur
Had Sulphur not being on Earth, life would have not been possible and the most evolved species known to the planet may not have even existed. When I say this, I am referring to man, homo-sapiens. The reason I say this is because in the early eons of the planets birth, simple chemical reactions involving Sulphur were able to take place in the seas. These reactions caused the productions of the planets first amino acids which were and till today are still the building blocks of everyday life.
In nature, Sulphur is naturally occurring near volcanoes. A large amount of natural Sulphur is found in the United States of America, namely Texas and Louisiana. Many Sulphide products that exist today have common names that do not necessarily contain the word Sulphide, therefore we do not fully respect the importance of Sulphur. A few examples of these include pyrite which is an iron sulphide, galena which is a lead sulphide, cinnabar which is a mercury sulphide, stibnite which is an antimony sulphide and even sphalerite which is a zinc sulphide. There also exists sulphide ores, which are considered more important than that of the aforementioned sulphide minerals. These ores include bornite, millerite, chalcopyrite, penlandite and many more. The main source of Sulphur is found in the natural gas hydrogen sulphide which goes through many extraction processes to eventually provide us with Sulphur. Canada is the chief producer of Sulphur in the world.
Environmental Effects
Although Sulphur may be an essential requirement for the human body, it does not benefit all living organisms to the same extent. Sulfur, which is not toxic, is found in the air we breathe but it is also accompanied by the harmful Sulphur compounds too. The occurrence of these negative compounds are produced during chemical reactions in industry. These Sulphur compounds cannot be easily “destroyed” and therefore industries release the gases produced into the air.
The damaging effects of Sulphur upon animals is one of extremities. Most commonly known is to affect the brain and cognitive abilities of the animal through damaging and malfunctioning of the hypothalamus and nervous system respectively. It can also be known to affect the hearts and kidneys, just as in humans, but has further damage in animals than humans. With animals, these toxins could cause genital and fetal damage also. Sulphur poisoning carried by the mother could be passed onto the offspring through ingestion of the mother’s milk. It is known to internally damage the enzyme system of animal also.
Additional Information
- Found on 0.06% of the Earths suraface (not very abundant)
- Most commonly found in its elemental form
- Largest reserves of Sulphur are found in sedimentary deposits
- Common forms of sulphur are soluble in carbon sulphide
- When heated, this solid melts and becomes a yellow-brownish vapour.
- Sulphur is insoluble in water
- When reacted and burnt in air, a blue flame is produced and it is converted to sulphur dioxide
Sulphur compounds
Periodic Table
There are many allotropic forms of Sulphur but the 2 most important types are rhombic and monoclinic with the former being the most stable form of Sulphur as it as a “puckered” ring structural formation.
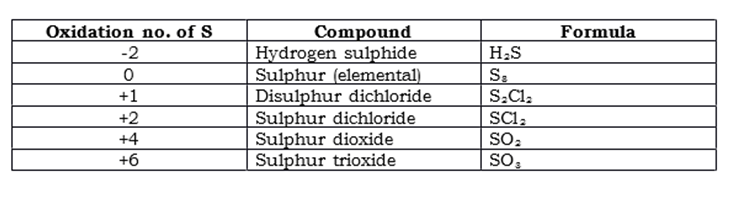
The famously known compound of Sulphur is hydrosulphuric acid, H2S. This is a colorless and toxic gas, that is soluble in alcohol as well as water, and is often referred to as having a “rotten egg” smell.
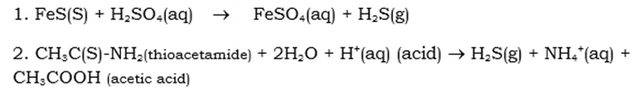
Laboratory Preperation is as follows:
There are 2 very important oxides to consider when dealing with Oxides, these are Sulphur Dioxide and Sulphur Trioxide, as mentioned in the table above.
Preparation of Sulphur dioxide is as follows:
- Industrial Preparation:

- Laboratory Preparation:
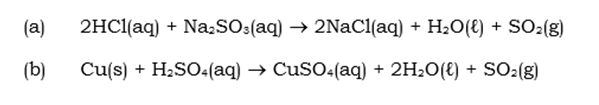
Reactions of Sulphur Dioxide:

The Contact Process- Preperation of Sulphuric Acid

The Contact Process
Step 1: Combustion of Sulphur:
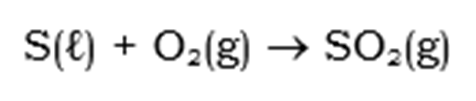
This reaction is a highly exothermic reaction and has an enthalpy of -298 kJ/mol.
Step 2: Conversion of Sulphur Dioxide (from Step 1) to Sulphur Trioxide:
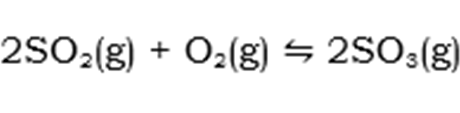
This is a catalytic reaction and has an enthalpy of -197 kJ/mol.
By carefully looking at Step 2, we apply Le Chatelier’s Principle. This Principle implies that the forward reaction is fovoured hence, the production of sulphur trioxide is favoured by the following conditions:
- Low Temperatures
- High Pressures
By this principle, logic tells us that the maximum equilibrium yield will be obtained by decreasing the temperature and decreasing the volume space of the reaction. However, when incoporating the economics of production, this is not the case.
In industry, if the temperature is lowered drastically, it will cause the reaction to be extremely slow and thus a low yield of Sulphur Trioxide will be produced. For high pressures to be obtained on site of a plant, specilized equipment will be need as well as high strenght materials. For those 2 requirements to be met which regards to pressure, the company will incur major costs. To overcome these problems, a compromise is made with regards to temperature and pressure. An "optimal" point is used. This is a point whereby maximum yield is produced by decreasing the temperature to a point that does not drastically slow down the reaction as well as a pressure that the equipment on site can handle, therefore eradicating extra costs to the company.
A catalyst, often used to increase the rate of the reaction, called Vanadium(v) Oxide is used. This process is referred to as the "Contact Process" due to Sulphur Dioxide and Oxygen moleules reacting when in contact with the catalyst, Vanadium(v) Oxide.
Step 3: Dissolving of Sulphuric Trioxide and production of Sulphuric Acid:
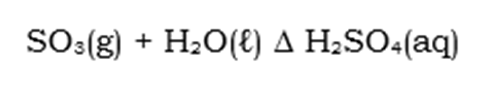
When conducting this reaction, certain precautionary measures should be addressed as it is a very violent reaction and injuries may occur if not conducted correctly as the reaction will produce a mist of Sulphuric Acid fumes that are corrosive.
To prevent injuries from occuring at all, a new method was inveneted. The Sulphuric Trioxide produced from Step 2 is now easily dissolved in a dilute Sulphuric Acid. Although this step is referred to as the production of sulfuric acid step, the dilute acid used is acid that was obtained from the previous conduction of the Contact Process. In other words, we need sulphuric acid to make sulphuric acid on a larger scale.
This sulphur trioxide is now an anhydride of sulphuric acid, and still has the same structural formulae. The reaction for this is as follows:
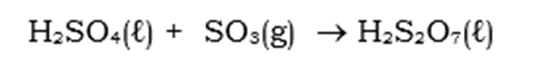
The liquid produced is called Oleum and and contains disulphuric acid. Upon cooling, the Oleum is mixed, once again, with diluted sulphuric acid to produce a concentrated sulphuric acid.
Uses of sulphuric acid include fertilizers, processing of ores containing metal, detergents, paints, and even as a catalyst in organic reactions
Images are linked to their sources in their description
The End
References:
[1]https://www.lenntech.com/periodic/elements/s.htm
[2]https://pubchem.ncbi.nlm.nih.gov/compound/hydrogen_sulfide
[3]https://www.britannica.com/technology/Frasch-process
[4]https://www.qld.gov.au/environment/pollution/monitoring/air-pollution/sulfur-dioxide
[5]https://www.chemguide.co.uk/physical/equilibria/contact.html
[6]www.rsc.org/periodic-table/element/16/sulfur




extraordinary post is very appropriate to be trending, hopefully I also one day later my post can be trending like you.
Thank you very much, yes i hope so too @suhadi-gayo, just keep working at it and you defintely will.
Congratulations @akeelsingh, this post is the fifth most rewarded post (based on pending payouts) in the last 12 hours written by a Dust account holder (accounts that hold between 0 and 0.01 Mega Vests). The total number of posts by Dust account holders during this period was 11360 and the total pending payments to posts in this category was $5806.15. To see the full list of highest paid posts across all accounts categories, click here.
If you do not wish to receive these messages in future, please reply stop to this comment.
Wow, i was unaware of this achievement. Thank you very much for this notification, it gives me joy knowing that people out there are enjoying my posts. I really appreciate it, thank you.
Informative
Thank you, i hope it has showed you the importance of Sulpur in our lives.