EXPLORING THE BIOCHEMISTRY OF GLYCATED HAEMOGLOBIN(HbA1c) FORMATION AND ANALYSIS USING THE ADVANCED HbA1c ANALYZER
The primary function of erythrocytes (red blood cells) is to carry oxygen to the tissues and to return carbon dioxide from these tissues to the lung for oxygenation.
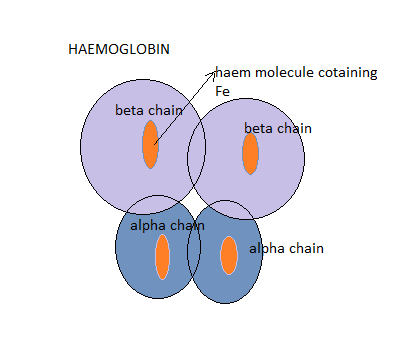
Haemoglobins are molecules found in the red blood cells and they are the majorly responsible for the transportation of oxygen to tissues and organ in the body. Each molecule of adult haemoglobin (HbA) is made up of four polypeptide chains of globins, 2Alpha chain and 2Beta chains with each chain having its own haem pocket.
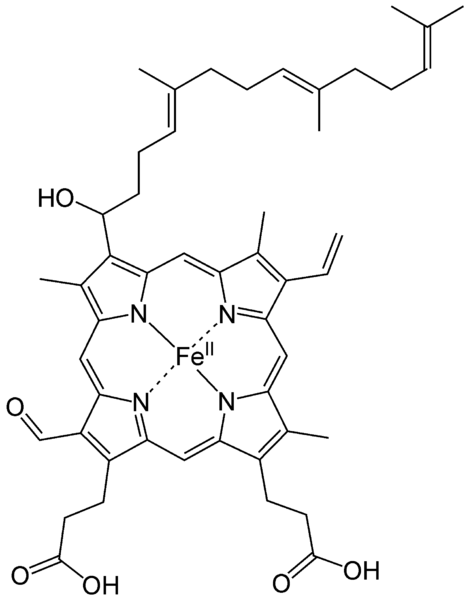
Haem is an iron formed when Glycine and Succinyl Coenzyme A in the presence of the enzyme Aminolaevulinic acid condenses to form a protoporphyrin molecule, this reaction requires a coenzyme pyridoxal phosphate (Vitamin B6). After a series of intermediate reactions, the resulting protoporphyrin molecule consequently combines with ferrous iron to form haem. The reaction between haem and globin chains leads to the formation of haemoglobin.
Glycine + SuccinylCoA = Protoporphyrin
Protoporphyrin~~~~~+ Fe2+ = Haem
Each of the globin chain has a pocket for the attachment of the haem molecule and it is in these pockets that oxygen is located.
The chains of globins interact and slide with one another during oxygenation and deoxygenation. During deoxygenation, these chains of globin pull apart or separate thereby allowing the entry of a molecule called 2,3-diphospoglycerate (2,3-DPG) which is responsible for regulating the affinity of these haemoglobin towards oxygen.
Entrance of 2,3-DPG reduces the affinity of haemoglobins towards oxygen. This is simply means that its moderate presence ensures that oxygen is released to tissues. In a situation where the concentration of these 2,3-DPG is higher than normal, oxygen is easily given off, for example in sickle cell anaemia. The reverse is the case in Fetal haemoglobin (HbF), this variant is found in fetus below 3-6months. haemoglobin F (HbF) does not bind 2,3-DPG and thus makes it very difficult for the haemoglobin molecule to give off or release oxygen easily.
Without the binding of oxygen to haemoglobin molecule, transportation of this oxygen to various tissues, organs and cells of the body would be very difficult.
Carbon monoxide (C0) binds reversibly to haemoglobin in a very stable form and in the process forms carboxyhaemoglobin. It easily displaces oxygen from haemoglobin and consequently makes oxygen unavailable for the haemoglobin.
The bond formed between carbon monoxide and haemoglobin is 200times stronger than that formed between haemoglobin and oxygen
This is why, when you inhale carbon monoxide accidentally, you shock, suffocate and gasp for air. Thus your rate of breathing is increased in order to take in enough oxygen that will replace those displaced by carbon monoxide from the haemoglobin.

How is this glycated haemoglobin formed?
As I earlier explained, Glycated haemoglobin simply means a haemoglobin molecule to which glucose residue is attached. This type of haemoglobin is mainly used to detect or know the average plasma glucose concentration over a long period of time. Glycation of haemoglbin occurs at the onset of life and thus, the indication here is that its measurement can be correctly used in the quantification of plasma glucose in the chronic diabetic patients.
The normal haemoglobin molecule in human has a paired B-chain which has an amine group called N-terminal valine and it is at this region that a glucose molecule binds and thus forms the glycated haemoglobin
The major form that does this is the HbA. Overtime this HbA becomes changed into HbA1 which also has some subtypes such as HbA1a, HbA1b and HbA1c. The HbA1c variant is what is referred to as glycated haemoglobin
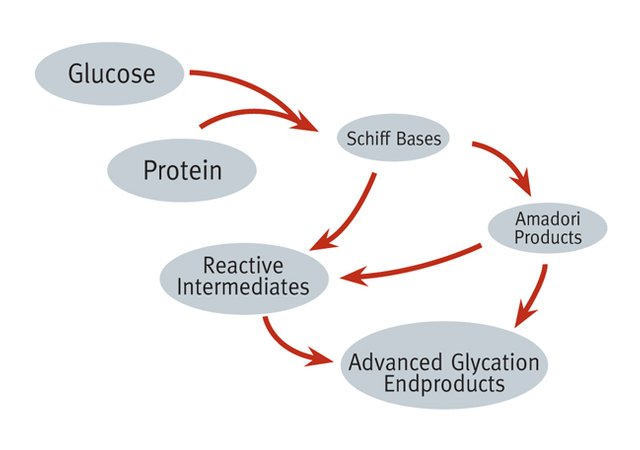
Glycated haemoglobin is usually formed invivo through a non-enzymatic reaction between excess glucose and a variant of haemoglobin molecule. It is formed through a process of glycation and what happens here is that, the excess plasma glucose in the body is bound to the N-terminal valine found on the B-chain of haemoglobin.
The binding of the glucose to the amino acids is a nucleophilic reactions and this does not require enzymes. The linkage of glucose to the N-terminal of the amino acid is very important because it gives rise to the changed or modified physical properties of the haemoglobin molecules and it is this changed properties that are mainly exploited during measurement.
After binding, the resultant product is the formation of a shift base adducts also known as Aldimine intermediate, this intermediate further undergo rearrangement through Amadori reaction to form a more stable HbA1c. The more glucose concentration in the body, the more glycated haemoglobin formed Glycation of haemoglobin occurs throughout the entire life span of red blood cells.
Glucose + haemoglobin = labile intermediate = Stable HbA1c
There are factors on which the formation of this glycosylated haemoglobin is dependent on. The major factors are time and concentration gradient.
The concentration of the glucose the haemoglobin is exposed to really play a significant role in the formation of HbA1c. Erythrocytes naturally have insulin independent glucose transporters which act as receptors on the red blood cell surface; they help in the free transportation of glucose across the semi permeable membrane of the red blood cell. This invariably means that when glucose levels in the plasma are high, then glucose levels inside the red blood cell will also be high. Consequently, the higher the glucose level, the more the glycosylation of haemoglobin will occur. It is logical to say here that glucose level in the blood determines HbA1c levels.
Secondly the duration of the blood glucose level in the blood is also a critical factor to be considered in the formation of glycated haemoglobin. The longer the blood glucose level is high, the more glycosylation will occur.
The red blood cells of Patients with disease conditions like sickle cell anaemia have naturally have a lower life span and thus glycation in these red blood cells will be very low compared to a an apparently healthy individual whose red blood life span averages 120days.

Why HbA1c measurement is important
Both plasma glucose levels in the body and glycated haemoglobin have a direct relationship. As the blood glucose level of the body increases, the rate of glycation also increase and as such, glycated haemoglobin estimations is vital tool to ascertain the risk of diabetes in humans. It is a pointer of impending diabetes and its
measurement represents the average blood glucose concentration in the body over the last 2-3 months of an individual. The results gotten can be used to monitor or access the efficacy of a therapy. Glycated haemoglobin measurements give an indication of chronic diabetes mellitus and its control.
The Glycated Haemoglbin analyser and how it works
The glycated haemoglobin anaalyser is an electronic machine that measures the concentration of glycosylated haemoglobin in the blood circulation. This machine can measure the concentration of glycated haemoglobin within three minutes!
This is faster than the normal conventional method of measuring fasting blood sugar. This method is much faster and easier because you don’t need to fast or undergo any special protocol before your blood sugar is measured.
This machine analyses HbA1c based on the electrical charge differences between HbA1c and other Hb molecules. It incorporates a High performance liquid chromatography in the estimation of glycated haemoglobin (HPLC).
The principle behind the operation of this machine is the fact that glycated haemoglobin has a very low isoelectric point and thus it migrates faster than other haemoglobin components when subjected to electric field.
The analyzer dilutes and haemolyses the blood with wash solution and subsequently injects small volume of the sample onto the HPLC column. The HbA1c are then separated based on their ionic interaction between the cation exchange molecules on the column resin surface. The next step is elution of the different variants of the HbA molecules using a buffer. After elution, the analyzer then passes these molecules through a photometer flow cell; at this point it measures the changes in absorbance at a wavelength of 415nmsource At this wavelength, glycated haemoglobin are detected
The results gotten are usually reported as percentage values and it corresponds to the percentage of the haemogoglobin that is glycated in the body. The method of reporting is now obsolete as the results gotten are now reported in millimoles per mol (mmol/mol). A result of these, 6% corresponds to 42mmol/mol, 6.5% corresponds to 48mmol/mol of glycated haemoglobin and so on.
The normal non diabetic glycated haemoglobin is in the range of 3.5-5.5%, values greater than 6.5% is an indication of diabetediabetes mellitus.
Are there factors that can limit HbA1c measurement?
In as much as HbA1c measurement is has enough benefit, its accurate measurement can be impeded by the presence of haemoglobin variants I.e haemoglobinopathies. E.g HbS, HbC, HbD and HbE, Hereditary persistent of HbF, Chemical derivatives of haemoglobin e.g Carbamylated Haemoglobin due to Uraemia and also Acetylated Haemoglobin due to large doses of aspirin. Alcoholism, Age, pregnancy, malaria, hemolytic anaemia, splenectomy etc also limit the measurement of HbA1c, they basically also lower the level of glycated haemoglobin.
This HbA1c method of estimation of plasma glucose concentration may not be so readily available in some countries due to its high cost. Erythropoiesis and red blood cell destruction also affect or limit the measurement of glycated haemoglobin using the analyzer.
How is plasma gluoce and glycosylated haemoglobin related?
Glycosylated haemoglobin and plasma glucose both have a linear and direct relationship.
It is worthy of note that, since red blood cells allows glucose to easily permeate through it, this simply implies that the rate of formation of glycosylated haemoglobin is directly proportional to the glucose concentration in the red blood cell circulating in the blood and it is also directly proportional to the duration or life span of the red blood cell and its turnover.
When plasma glucose level in the body is normal, there is also normal level of glycated haemoglobin. As the average amount of the plasma blood glucose increases, the amount of or fraction of glycated haemoglobin also increases. This is why glycated haemogblobin serves as a good indicator of the increase in blood glucose level.
Conclusion
On the average, we can conclusively say that Glycosylated hemoglobin of about 6% is equal or corresponds to mean plasma glucose of 135mg/dl. What this implies is that, for every 1% increase in the glycosylated haemoglobin, the mean plasma glucose concentration increases by 35mg/dl.
It is safe to say that, glycated haemoglobin measurement using the HbA1c analyzer is a faster way of monitoring a chronic diabetic patient when compared to the normal method of fasting blood sugar estimation. The level of HbA1c helps the clinician to easily know when a complication begins and it's variability among individuals is very minimal.
thanks for reading

references
•werlabs-diabetes
•glycated haemoglobin test
•glycated hba1c measurement
•ncbi- glycated haemoglobin
•cdc-lab procedure manual
•WHO-use of glycated haemoglobin
•Karen Reed, (2014). The HbA1c test. Www.daibetesinfo.org.nz accessed may 25 2018. 01:20GMT.
•Konstantinos Makes and Loukia Spanou, (2011). Is there a relationship between mean blood glucose and glycated haemoglobin. Journal of diabetes Science and Technology. Vol.5, issue 6.
•Randie R. Little and Roberts M.D. (2009). A review of variant Haemoglobins interfering with Haemoglobin A1c measurement. Journal of Diabetes and science technology


Congratulations! this post got an upvote by @steemrepo and was manually picked by the curator @yanosh01 to be added on STEEM REPOSITORY, simply comment "YES" and we upload it on STEEM REPO Website.
Want to know more about the Steem Repo project? Contact us on Discord
Yes
Keep up the good work, I like your articles and they are well structured.
You are also very active around. Perhaps having a conclusion or conclusions would help, remember that a Curie vote requires you to have a conclusion.
As for the biochemistry, I admit I am a little overwhelmed by the details but I am always open to read more! I know just enough as to read my blood test results even before I get to the doctor :D
Thanks bro for this lovely response. Has been taken into consideration.
I guess I'll be so science inclined just through your posts.
Hopefully 🤗
Thanks for sharing this post....very educative....well done bro.
💖
Wonderful piece of writing...i know this would have taken you up to a month to arrange...anyways quality is what we are looking for and your piece of work got that quality
I highly appreciate your comment.
🙌
In terms of quality and quantity, I would give you a hundred plus! Well done dear
Thanks dear