Summary
Following up on a previous post focused on celiac disease, triggered by ingestion of wheat gluten in genetically susceptible individuals (see Is Bread Certain Death?), I'd like to begin to talk about other autoimmune diseases. Here I will focus on the prevalance of autoimmune diseases and their associations with the major histocompatibility complex (MHC) as primary genetic risk factors. In subsequent articles I propose to discuss potential causes, environmental triggers, roles of the Western diet and hygiene, relationships to the microbiome and infectious agents, pathology, autoantibodies, roles of T and B cells, secondary genetic risk factors (outside of the MHC), and progress in development of treatments for specific autoimmune diseases.
Prevalence of Autoimmune Diseases
Estimates of the prevalence of autoimmune diseases are presented by the Autoimmune Registry, relying mostly on an article published by Hayter and Cook (2012), who concluded that about 14.7 million people in the U.S. were affected by at least one of 81 autoimmune diseases. The Autoimmune Registry lists the following 26 autoimmune diseases exceeding a prevalance of >0.007 percent in the U.S. population:
- Rheumatoid arthritis: 0.806%
- Hashimoto's autoimmune thyroiditis: 0.742%
- Celiac disease: 0.703%
- Graves' disease: 0.590%
- Type 1 diabetes: 0.450%
- Vitiligo: 0.375%
- Rheumatic fever: 0.234%
- Pernicious anemia/atrophic gastritis: 0.141%
- Alopecia areata: 0.1412%
- Immune thrombocytopenic purpura: 0.068%
- Multiple sclerosis: 0.055%
- Systemic lupus erythematosus: 0.030%
- Temporal arteritis: 0.028%
- Ulcerative colitis: 0.028%
- Crohn's disease: 0.023%
- Scleroderma: 0.023%
- Antiphospholipid syndrome: 0.020%
- Autoimmune hepatitis, type 1: 0.015%
- Primary biliary cirrhosis/cholangitis: 0.014%
- Sjogren's syndrome: 0.014%
- Addison's disease: 0.013%
- Dermatitis herpetiformis: 0.011%
- Kawasaki disease 0.009%
- Sympathetic opthalmia: 0.008%
- HLA-B27 associated anterior uveitis: 0.008%
- Primary sclerosing cholangitis: 0.008%
This data is likely incomplete as it does not include psoriasis, affecting 3.2% of the adult population in the U.S. (Rachakonda et al. (2014)), psoriatic arthritis, affecting between 0.1 to 0.16% of the US population (Reveille (2011), Liu et al. 2014)), and ankylosing spondylitis, affecting between 0.2 to 0.5% of the U.S. population (Reveille (2011)).
If we include these addiitional diseases of relatively high prevalance, the true number of persons affected by autoimmune diseases in the U.S. population is likely closer to 23.5 million, a number cited in the book "The Autoimmune Diseases (Fifth Edition)", and by the National Institutes of Health in their brochure on "Autoimmune Diseases".
Assuming a U.S. population of 327 million, this would mean about 7.2% of the U.S. population is affected by one or more autoimmune diseases. This value is close to the estimated prevalence of autoimmune diseases in Denmark (7.6 - 9.4%) (Cooper et al. (2009)).
Alarmingly, the prevalence of autoimmune diseases is not static but seems to be on the increase world-wide (Lerner et al. (2015)). The reasons for this will be the subject of a future article. At the moment, suffice it to say that this implies a strong environmental component to the onset of autoimmune diseases, super-imposed on a fundamental genetic susceptibility ("The Autoimmune Diseases (Fifth Edition)").
The Major Histocompatibility Complex and Autoimmune Disease Susceptibility
It is widely recognized that autoimmune diseases tend to be familial, and that the principal genetic risk factors for autoimmune diseases reside in the Major Histocompatibility Complex on human chromosome 6.
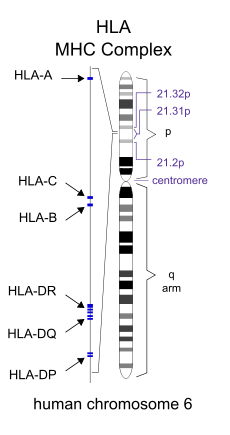
Major Histocompatibility Complex - Human Leukocyte Antigen (HLA) region of Chromosome 6. Image Source:
Horton et al. (2004) have published a more complete gene map of the extended human major histocompatibility complex. available in poster (.pdf) format: "The extended major histocompatibility complex (xMHC) on chromosome 6 is essential for adaptive and innate immunity. In addition to their vital role in transplant medicine, certain combinations (haplotypes) of xMHC loci are known to confer protection from, or susceptibility to, many diseases including most, if not all, autoimmune, inflammatory and infectious diseases. Paralleled only by war and famine, these diseases rank as the leading cause of human mortality and disability worldwide. History has shown that knowledge of detailed and accurate genetic maps enhances our ability to diagnose, understand and treat disease at the molecular level" (Horton et al. (2004)). Stewart et al. (2004) have further presented the complete sequence of two common haplotypes of the MHC to which over 100 diseases have been mapped.
Listed below are copious examples of some of the associations that have been recognized between different genes and their alleles (gene variants) within the MHC and specific autoimmune diseases, drawn from a survey of the primary literature conducted over the last week. For each gene within the MHC listed below, I have not specified which allele is implicated in each disease. I have, however, provided a link to the NCBI-NLM-NIH Gene entry for each locus (or pair of loci) which gives a summary of the function(s) of each gene and a detailed bibliography for each gene:
HLA-G major histocompatibility complex, class I, G: Multiple sclerosis (Cree et al. (2010)); Systemic lupus erythematosus (Lee et al. (2015), Lee and Bae (2015))
HLA-A major histocompatibility complex, class I, A: Psoriasis (Hirata et al.(2018)); Behçet's disease (Akpolat et al. (1992)); Type 1 diabetes (Gough and Simmonds (2007)); Birdshot chorioretinopathy (Hou et al. (2015))
HLA-C major histocompatibility complex, class I, C: Psoriasis (Hirata et al.(2018), Cardili et al. (2016)); Psoriatic arthritis (Hüffmeier and Mössner (2014)); Multiple sclerosis (Yeo et al. (2007), Gough and Simmonds (2007)); Primary sclerosing cholangitis (Hov et al. (2010)); Graves' disease (Gough and Simmonds (2007), Simmonds et al. (2007))
HLA-B major histocompatibility complex, class I, B: Primary sclerosing cholangitis (Næss et al. (2014)); Spondyloarthritis (Reveille (2004), Bodis et al. (2018)); Behçet's disease (Bodis et al. (2018); Pirim et al. (2014)); Myasthenia gravis (Varade et al. (2018)); Addison's disease (Skinningsrud et al. (2011)); Sarcoid arthropathy (Petursdottir et al. (2013)); Type 1 diabetes (Gough and Simmonds (2007)); Ankylosing spondylitis (Gough and Simmonds (2007)); Graves' disease (Gough and Simmonds (2007), Simmonds et al. (2007)); Acute anterior uveitis (Hou et al. (2015))
MICA MHC class I polypeptide-related sequence A/MICB MHC class I polypeptide-related sequence B: Sjögren's syndrome (Carapito et al. (2017)); Primary sclerosing cholangitis (Wiencke et al. (2001)); Addison's disease (Skinningsrud et al. (2011)); Type 1 diabetes (Gough and Simmonds (2007)); Rheumatoid arthritis (Gough and Simmonds (2007)); Psoriasis (Song et al. (2014)); Psoriatic arthritis (Song et al. (2014)); Systemic lupus erythematosus (Morris et al. (2012))
TNFA tumor necrosis factor (TNF-alpha): Myasthenia gravis (Skeie et al. (1999)); Multiple sclerosis (Rahmanian and Kargar (2014)); Idiopathic intermediate uveitis (Atan et al. (2013)); Rheumatoid arthritis (Gough and Simmonds (2007)); Graves' disease (Gough and Simmonds (2007), Morita et al. (2018)); Hashimoto's thyroiditis (Morita et al. (2018)); Systemic lupus erythematosus (Gough and Simmonds (2007)); Juvenile idiopathic arthritis (Schmeling et al. (2006)); Psoriasis (Loft et al. (2016), Cardili et al. (2016)); Psoriatic arthritis (Murdaca et al. (2014))
C4A complement C4A/C4B complement C4B: Systemic lupus erythematosus (Gough and Simmonds (2007), Tsang-A-Sjoe et al. (2017)); Crohn's disease (Cleynen et al. (2016)); Juvenile dermatomyositis (Lintner et al. (2016)); Behçet's disease (Hou et al. (2015)
NOTCH4 notch 4: Primary sclerosing cholangitis (Næss et al. (2014)); Alopecia areata (Tazi-Ahnini et al. (2003)); Systemic lupus erythematosus (Morris et al. (2012))
BTNL2 butyrophilin like 2: Sarcoidosis (Morais et al. (2012)); Ulcerative colitis (Pathan et al. (2009)); Grave's disease (Gough and Simmonds (2007)); Type 1 diabetes (Orozco et al. (2005)); Rheumatoid arthritis (Orozco et al. (2005)); Systemic lupus erythematosus (Orozco et al. (2005)); Sporadic inclusion body myositis (Scott et al. (2011))
HLA-DRA major histocompatibility complex, class II, DR alpha/HLA-DRB1 major histocompatibility complex, class II, DR beta 1: Multiple sclerosis (Gianfrancesco et al. (2017), Gough and Simmonds (2007)); Primary sclerosing cholangitis (Næss et al. (2014)); Autoimmune hepatitis (Junge et al. (2016)); Ulcerative colitis (Goyette et al. (2015); Crohn's disease (Goyette et al. (2015)); Ankylosing spondilitis (Perez-Guijo (2002)); Autoimmune thyroid diseases (Hashimoto's thyroiditis and Graves' disease) (Tomer (2010), Ramgopal et al. (2018), Gough and Simmonds (2007), Simmonds et al. (2007)); Alopecia areata (Betz et al. (2015)); Rheumatoid arthritis (Bodis et al. (2018), de Vries et al. (2005), Gough and Simmonds (2007)); Behçet's disease (Pirim et al. (2014)); Primary biliary cirrhosis/cholangitis (Gulamhusein et al. (2015), Li et al. (2014)); Antiphospholipid syndrome (Sebastiani et al. (2016)); Sjögren's syndrome (Huang et al. (2015)); Myasthenia gravis (Popperud et al. (2017), Gough and Simmonds (2007)); Addison's disease (Skinningsrud et al. (2011)); Sarcoidosis (Levin et al. (2015)); Type 1 diabetes (Gough and Simmonds (2007)); Systemic lupus erythematosus (Gough and Simmonds (2007)); Autoimmune polyglandular syndrome type II (Weinstock et al. (2011)); Autoimmune polyglandular syndrome type III (Hashimoto et al. (2005)); Vogt-Koyanagi-Harada syndrome (Hou et al. (2015)); Sporadic inclusion body myositis (Scott et al. (2011))
HLA-DQA1 major histocompatibility complex, class II, DQ alpha 1/HLA-DQB1 major histocompatibility complex, class II, DQ beta 1: Psoriasis vulgaris (Hirata et al.(2018); Systemic lupus erythematosus (Sun et al. (2018)); Celiac disease (Megiorni and Pizzuti (2012), Bodis et al. (2018), Gough and Simmonds (2007)); Primary biliary cirrhosis/cholangitis (Gulamhusein et al. (2015)); Antiphospholipid syndrome (Namjou (2003)); Sjögren's syndrome (Cruz-Tapias et al. (2012)); Myasthenia gravis (Yousefipour et al. (2009)); Type 1 diabetes (Gough and Simmonds (2007)); Autoimmune polyglandular syndrome type III (Hashimoto et al. (2005)); Graves' disease (Simmonds et al. (2007)); Vogt-Koyanagi-Harada syndrome (Hou et al. (2015)); Autoimmune polyglandular syndrome type II (Weinstock et al. (2011))
TAP1 transporter 1, ATP binding cassette subfamily B member/TAP2 transporter 2, ATP binding cassette subfamily B member: Systemic sclerosis (Song et al. (2005))
HLA-DMA major histocompatibility complex, class II, DM alpha/HLA-DMB major histocompatibility complex, class II, DM beta: Antiphospholipid syndrome (Sanchez et al. (2004))
HLA-DPA1 major histocompatibility complex, class II, DP alpha 1/HLA-DPB1 major histocompatibility complex, class II, DP beta 1: Wegener's granulomatosis (Merkel et al. (2017)); Rheumatoid arthritis (Huang et al. (2018), Jiang et al. (2018)); Primary biliary cirrhosis/cholangitis (Gulamhusein et al. (2015)); Chronic beryllium disease (Dai et al. (2010), Alvaro-Benito et al. (2016))
RXRB retinoid X receptor beta: Systemic sclerosis (Oka et al. (2017)); Wegener's granulomatosis (Wieczorek et al. (2009), Szyld et al. (2006))
Please note that all of these genes are confined to a relatively small region of the short-arm of chromosome 6. Many additional (non-HLA) genes, scattered over the remainder of the human genome have also been identified to confer susceptibility or resistance to autoimmune diseases, but these are best discussed when we consider individual autoimmune diseases in future articles.
References
Akpolat, T., Koç, Y., Yeniay, I., Akpek, G., Güllü, I. et al. Familial Behçet's disease. Eur. J. Med. 1: 391-395 (1992)
Alvaro-Benito, M., Morrison, E., Wieczorek, M., Sticht, J., Freund, C. Human leukocyte antigen-DM polymorphisms in autoimmune diseases. Open Biol. 6 pii: 160165 (2016)
Atan, D., Heissigerova, J., Kuffová, L., Hogan, A., Kilmartin, D.J., Forrester, J.V., Bidwell, J.L., Dick, A.D., Churchill, A.J. Tumor necrosis factor polymorphisms associated with tumor necrosis factor production influence the risk of idiopathic intermediate uveitis. Mol. Vis. 19: 184-195 (2013)
Betz, R.C., Petukhova, L., Ripke, S., Huang, H., et al. Genome-wide meta-analysis in alopecia areata resolves HLA associations and reveals two new susceptibility loci. Nat. Commun. 6: 5966 (2015)
Bodis, G., Toth, V., Schwarting, A. Role of human leukocyte antigens (HLA) in autoimmune diseases. Rheumatol. Ther. 5: 5-20 (2018)
Carapito, R., Gottenberg, J.E., Kotova, I., Untrau, M., et al. A new MHC-linked susceptibility locus for primary Sjögren's syndrome: MICA. Hum. Mol. Genet. 26: 2565-2576 (2017)
Cardili, R.N., Deghaide, N.S., Mendes-Junior, C.T., Donadi, E.A., Souza, C.S. HLA-C and TNF gene polymorphisms are associated with psoriasis in Brazilian patients. Int. J. Dermatol. 55: e16-e22 (2016)
Cleynen, I., Konings, P., Robberecht, C., Laukens, D., Amininejad, L., Théâtre, E., Machiels, K., Arijs, I., Rutgeerts, P., Louis, E., Franchimont, D., De Vos, M., Van Steen, K., Georges, M., Moreau, Y., Vermeesch, J., Vermeire, S. Genome-wide copy number variation scan identifies complement component C4 as novel susceptibility gene for Crohn's disease. Inflamm. Bowel Dis. 22: 505-515 (2016)
Cooper, G.S., Bynum, M.L., Somers, E.C. Recent insights in the epidemiology of autoimmune diseases: improved prevalence estimates and understanding of clustering of diseases. J. Autoimmun. 33: 197-207 (2009)
Cree, B.A., Rioux, J.D., McCauley, J.L., Gourraud, P.A., et al. A major histocompatibility class I locus contributes to multiple sclerosis susceptibility independently from HLA-DRB1-15:01. PLoS One 5: e11296 (2010)
Cruz-Tapias, P., Rojas-Villarraga, A., Maier-Moore, S., Anaya, J.M. HLA and Sjögren's syndrome susceptibility. A meta-analysis of worldwide studies. Autoimmun. Rev. 11: 281-287 (2012)
Dai, S., Murphy, G.A., Crawford, F., Mack, D.G., Falta, M.T., Marrack, P., Kappler, J.W., Fontenot, A.P. Crystal structure of HLA-DP2 and implications for chronic beryllium disease. Proc. Natl. Acad. Sci. U.S.A. 107: 7425-7430 (2010)
de Vries, R.R., Huizinga, T.W., Toes, R.E. Redefining the HLA and RA association: to be or not to be anti-CCP positive. J. Autoimmun. 25 Suppl: 21-25 (2005)
Gianfrancesco, M.A., Stridh, P., Shao, X., Rhead, B., et al. Genetic risk factors for pediatric-onset multiple sclerosis. Mult. Scler. (Epub ahead of print) (2017)
Gough, S.C., Simmonds, M.J. The HLA region and autoimmune disease: associations and mechanisms of action. Curr. Genomics. 8: 453-465 (2007)
Goyette, P., Boucher, G., Mallon, D., Ellinghaus, E., Jostins, L., Huang, H., et al. High-density mapping of the MHC identifies a shared role for HLA-DRB1-01:03 in inflammatory bowel diseases and heterozygous advantage in ulcerative colitis. Nat. Genet. 47: 172-179 (2015)
Gulamhusein, A.F., Juran, B.D., Lazaridis, K.N. Genome-wide association studies in primary biliary cirrhosis. Semin. Liver Dis. 35: 392-401 (2015)
Hashimoto, K., Maruyama, H., Nishiyama, M., Asaba, K., Ikeda, Y., Takao, T., Iwasaki, Y., Kumon, Y., Suehiro, T., Tanimoto, N., Mizobuchi, M., Nakamura, T. Susceptibility alleles and haplotypes of human leukocyte antigen DRB1, DQA1, and DQB1 in autoimmune polyglandular syndrome type III in Japanese population. Horm. Res. 64: 253-260 (2005)
Hayter, S.M., Cook, M.C. Updated assessment of the prevalence, spectrum and case definition of autoimmune disease. Autoimmun. Rev. 11: 754-765 (2012)
Hirata, J., Hirota, T., Ozeki, T., Kanai, M., Sudo, T., Tanaka, T., Hizawa, N., Nakagawa, H., Sato, S., Mushiroda, T., Saeki, H., Tamari, M., Okada, Y. Variants at HLA-A, HLA-C, and HLA-DQB1 confer risk of psoriasis vulgaris in Japanese. J. Invest. Dermatol. 138: 542-548 (2018)
Horton, R., Wilming, L., Rand, V., Lovering, R.C., Bruford, E.A., Khodiyar, V.K., Lush, M.J., Povey, S., Talbot, C.C. Jr., Wright, M.W., Wain, H.M., Trowsdale, J., Ziegler, A., Beck, S. Gene map of the extended human MHC. Nat. Rev. Genet. 5: 889-899 (2004)
Hou, S., Kijlstra, A., Yang, P. Molecular genetic advances in uveitis. Prog. Mol. Biol. Transl. Sci. 134: 283-298 (2015)
Hov, J.R., Lleo, A., Selmi, C., Woldseth, B., Fabris, L., Strazzabosco, M., Karlsen, T.H., Invernizzi. P. Genetic associations in Italian primary sclerosing cholangitis: heterogeneity across Europe defines a critical role for HLA-C. J. Hepatol. 52: 712-717 (2010)
Huang, R., Yin, J., Chen, Y., Deng, F., Chen, J., Gao, X., Liu, Z., Yu, X., Zheng, J. The amino acid variation within the binding pocket 7 and 9 of HLA-DRB1 molecules are associated with primary Sjögren's syndrome. J. Autoimmun. 57: 53-59 (2015)
Huang, Z., Niu, Q., Yang, B., Zhang, J., Yang, M., et al. Genetic polymorphism of rs9277535 in HLA-DP associated with rheumatoid arthritis and anti-CCP production in a Chinese population. Clin. Rheumatol. (Epub ahead of print) (2018)
Hüffmeier, U., Mössner, R. Complex role of TNF variants in psoriatic arthritis and treatment response to anti-TNF therapy: evidence and concepts. J. Invest. Dermatol. 134: 2483-2485 (2014)
Jiang, L., Jiang, D., Han, Y., Shi, X., Ren, C. Association of HLA-DPB1 polymorphisms with rheumatoid arthritis: a systemic review and meta-analysis. Int. J. Surg. 52: 98-104 (2018)
Junge, N., Tiedau, M., Verboom, M., Hallensleben, M., Blasczyk, R., Schlue, J., Goldschmidt, I., Pfister, E.D., Baumann, U. Human leucocyte antigens and pediatric autoimmune liver disease: diagnosis and prognosis. Eur. J. Pediatr. 175: 527-537 (2016)
Lee, Y.H., Bae, S.C. Association between a functional HLA-G 14-bp insertion/deletion polymorphism and susceptibility to autoimmune diseases: a meta-analysis. Cell. Mol. Biol. (Noisy-le-grand) 61: 24-30 (2015)
Lee, Y.H., Bae, S.C., Song, G.G. Meta-analysis of associations between functional HLA-G polymorphisms and susceptibility to systemic lupus erythematosus and rheumatoid arthritis. Rheumatol. Int. 35: 953-961 (2015)
Lerner, A., Jeremias, P., Matthias, T. The world incidence and prevalence of autoimmune diseases is increasing. International Journal of Celiac Disease 3: 151-155 (2015)
Levin, A.M., Adrianto, I., Datta, I., Iannuzzi, M.C., Trudeau, S., Li, J., Drake, W.P., Montgomery, C.G., Rybicki, B.A. Association of HLA-DRB1 with sarcoidosis susceptibility and progression in African Americans. Am. J. Respir. Cell. Mol. Biol. 53: 206-216 (2015)
Li, M., Zheng, H., Tian, Q.B., Rui, M.N., Liu, D.W. HLA-DR polymorphism and primary biliary cirrhosis: evidence from a meta-analysis. Arch. Med. Res. 45: 270-279 (2014)
Lintner, K.E., Patwardhan, A., Rider, L.G., Abdul-Aziz, R., Wu, Y.L., Lundström, E., Padyukov, L., Zhou, B., Alhomosh, A., Newsom, D., White, P., Jones, K.B., O'Hanlon, T.P., Miller, F.W., Spencer, C.H., Yu, C.Y. Gene copy-number variations (CNVs) of complement C4 and C4A deficiency in genetic risk and pathogenesis of juvenile dermatomyositis. Ann. Rheum. Dis. 75: 1599-1606 (2016)
Liu, J.T., Yeh, H.M., Liu, S.Y., Chen, K.T. Psoriatic arthritis: epidemiology, diagnosis, and treatment. World J. Orthop. 5: 537–543 (2014)
Loft, N.D., Skov, L., Rasmussen, M.K., Gniadecki, R., et al. Genetic polymorphisms associated with psoriasis and development of psoriatic arthritis in patients with psoriasis. PLoS One 13: e0192010 (2018)
Megiorni, F., Pizzuti, A. HLA-DQA1 and HLA-DQB1 in Celiac disease predisposition: practical implications of the HLA molecular typing. J. Biomed. Sci. 19: 88 (2012)
Merkel, P.A., Xie, G., Monach, P.A., Ji, X., Ciavatta, D.J., et al. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 69: 1054-1066 (2017)
Morais, A., Lima, B., Peixoto, M.J., Alves, H., Marques, A., Delgado, L. BTNL2 gene polymorphism associations with susceptibility and phenotype expression in sarcoidosis. Respir. Med. 106: 1771-1777 (2012)
Morris, D.L., Taylor, K.E., Fernando, M.M., Nititham, J., et al. Unraveling multiple MHC gene associations with systemic lupus erythematosus: model choice indicates a role for HLA alleles and non-HLA genes in Europeans. Am. J. Hum. Genet. 91: 778-793 (2012)
Morita, E., Watanabe, M., Inoue, N., Hashimoto, H., Haga, E., Hidaka, Y., Iwatani, Y. Methylation levels of the TNFA gene are different between Graves' and Hashimoto's diseases and influenced by the TNFA polymorphism. Autoimmunity (Epub ahead of print) (2018)
Murdaca, G., Gulli, R., Spanò, F., Lantieri, F., Burlando, M., Parodi, A., Mandich, P., Puppo, F. TNF-alpha gene polymorphisms: association with disease susceptibility and response to anti-TNF-alpha treatment in psoriatic arthritis. J. Invest. Dermatol. 134: 2503-2509 (2014)
Næss, S., Lie, B.A., Melum, E., Olsson, M., Hov, J.R., et al. Refinement of the MHC risk map in a scandinavian primary sclerosing cholangitis population. PLoS One 9: e114486 (2014)
Namjou, B. Antiphospholipid syndrome: genetic review. Curr. Rheumatol. Rep. 5: 391-394 (2003)
Oka, A., Asano, Y., Hasegawa, M., Fujimoto, M., et al. RXRB is an MHC-encoded susceptibility gene associated with anti-topoisomerase I antibody-positive systemic sclerosis. J. Invest. Dermatol. 137: 1878-1886 (2017)
Orozco, G., Eerligh, P., Sánchez, E., Zhernakova, S., et al. Analysis of a functional BTNL2 polymorphism in type 1 diabetes, rheumatoid arthritis, and systemic lupus erythematosus. Hum. Immunol. 66: 1235-1241 (2005)
Pandey, J.P., Takeuchi, F. TNF-alpha and TNF-beta gene polymorphisms in systemic sclerosis. Hum. Immunol. 60: 1128-1130 (1999)
Pathan, S., Gowdy, R.E., Cooney, R., Beckly, J.B., Hancock, L., Guo, C., Barrett, J.C., Morris, A., Jewell, D.P. Confirmation of the novel association at the BTNL2 locus with ulcerative colitis. Tissue Antigens 74: 322-329 (2009)
Perez-Guijo, V., Muñoz, E., Escudero, A., Veroz, R., Sánchez, M., Muñoz-Villanueva, M.C., González, R., Peña, J., Collantes-Estevez, E. Distribution of HLA-DRB1 genes in patients with sporadic ankylosing spondylitis in the south of Spain. Joint Bone Spine 69: 458-462 (2002)
Petursdottir, D., Haraldsdottir, S.O., Bjarnadottir, K., Jonsson, T., Gislason, T., Gudmundsson, S., Gudbjornsson, B. Sarcoid arthropathy and the association with the human leukocyte antigen. The Icelandic Sarcoidosis Study. Clin. Exp. Rheumatol. 31: 711-716 (2013)
Pirim, I., Atasoy, M., Ikbal, M., Erdem, T., Aliagaoglu, C. HLA class I and class II genotyping in patients with Behcet's disease: a regional study of eastern part of Turkey. Tissue Antigens 64: 293-297 (2004)
Popperud, T.H., Viken, M.K., Kerty, E., Lie, B.A. Juvenile myasthenia gravis in Norway: HLA-DRB1-04:04 is positively associated with prepubertal onset. PLoS One 12: e0186383 (2017)
Rachakonda, T.D., Schupp, C.W., Armstrong, A.W. Psoriasis prevalence among adults in the United States. J. Am. Acad. Dermatol. 70: 512-516 (2014)
Rahmanian, M., Kargar, M. Tumor necrosis factor-alpha polymorphism and susceptibility to multiple sclerosis in the Iranian population. Iran Red Crescent Med. J. 17: e18247 (2014)
Ramgopal, S., Rathika, C., Padma, M.R., Murali, V., Arun, K., Kamaludeen, M.N., Balakrishnan, K. Interaction of HLA-DRB1 alleles and CTLA4 (+49 AG) gene polymorphism in autoimmune thyroid disease. Gene 642: 430-438 (2018)
Reveille, J.D. The genetic basis of spondyloarthritis. Curr. Rheumatol. Rep. 6: 117-125 (2004)
Reveille, J.D. Epidemiology of spondyloarthritis in North America. Am. J. Med. Sci. 341: 284–286 (2011)
Sanchez, M.L., Katsumata, K., Atsumi, T., Romero, F.I., Bertolaccini, M.L., Funke, A., Amengual, O., Kondeatis, E., Vaughan, R.W., Cox, A., Hughes, G.R., Khamashta, M.A. Association of HLA-DM polymorphism with the production of antiphospholipid antibodies. Ann. Rheum. Dis. 63: 1645-1648 (2004)
Schmeling, H., Wagner, U., Peterson, A., Horneff, G. Tumor necrosis factor alpha promoter polymorphisms in patients with juvenile idiopathic arthritis. Clin. Exp. Rheumatol. 24: 103-108 (2006)
Scott, A.P., Laing, N.G., Mastaglia, F., Needham, M., Walter, M.C., Dalakas, M.C., Allcock, R.J. Recombination mapping of the susceptibility region for sporadic inclusion body myositis within the major histocompatibility complex. J. Neuroimmunol. 235: 77-83 (2011)
Sebastiani, G.D., Iuliano, A., Cantarini, L., Galeazzi, M. Genetic aspects of the antiphospholipid syndrome: an update. Autoimmun. Rev. 15: 433-439 (2016)
Simmonds, M.J., Howson, J.M., Heward, J.M., Carr-Smith, J., Franklyn, J.A., Todd, J.A., Gough, S.C. A novel and major association of HLA-C in Graves' disease that eclipses the classical HLA-DRB1 effect. Hum. Mol. Genet. 16: 2149-2153 (2007)
Skeie, G.O., Pandey, J.P., Aarli, J.A., Gilhus, N.E. TNFA and TNFB polymorphisms in myasthenia gravis. Arch. Neurol. 56: 457-461 (1999)
Skinningsrud, B., Lie, B.A., Lavant, E., Carlson, J.A., Erlich, H., Akselsen, H.E., Gervin, K., Wolff, A.B., Erichsen, M.M., Løvås, K., Husebye, E.S., Undlien, D.E. Multiple loci in the HLA complex are associated with Addison's disease. J. Clin. Endocrinol. Metab. 96: E1703-E1708 (2011)
Song, G.G., Kim, J.H., Lee, Y.H. Associations between the major histocompatibility complex class I chain-related gene A transmembrane (MICA-TM) polymorphism and susceptibility to psoriasis and psoriatic arthritis: a meta-analysis. Rheumatol. Int. 34: 117-123 (2014)
Song, Y.W., Lee, E.B., Whang, D.H., Kang, S.J., Takeuchi, F., Park, M.H. Association of TAP1 and TAP2 gene polymorphisms with systemic sclerosis in Korean patients. Hum. Immunol. 66: 810-817 (2005)
Stewart, C.A., Horton, R., Allcock, R.J., Ashurst, J.L., Atrazhev, A.M., et al. Complete MHC haplotype sequencing for common disease gene mapping. Genome Res. 14: 1176-1187 (2004)
Sun, J., Yang, C., Fei, W., Zhang, X., Sheng, Y., Zheng, X., Tang, H., Yang, W., Yang, S., Fan, X., Zhang, X. HLA-DQB1 amino acid position 87 and DQB1-0301 are associated with Chinese Han SLE. Mol. Genet. Genomic Med. (Epub ahead of print) (2018)
Szyld, P., Jagiello, P., Csernok, E., Gross, W.L., Epplen, J.T. On the Wegener granulomatosis associated region on chromosome 6p21.3. BMC Med. Genet. 7: 21 (2006)
Tazi-Ahnini, R., Cork, M., Wengraf, D., Wilson, A.G., Gawkrodger, D.J., Birch, M.P., Messenger, A.G., McDonagh, A.J.Notch4, a non-HLA gene in the MHC is strongly associated with the most severe form of alopecia areata. Hum. Genet. 112: 400-403 (2003)
The Autoimmune Diseases (Fifth Edition). Edited by: Ian R. Mackay and Noel Richard Rose. ISBN: 978-0-12-384929-8
Tomer, Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid 20: 715-725 (2010)
Tsang-A-Sjoe, M.W.P., Bultink, I.E.M., Korswagen, L.A., van der Horst, A., Rensink, I., de Boer, M., Hamann, D., Voskuyl, A.E., Wouters, D. Comprehensive approach to study complement C4 in systemic lupus erythematosus: gene polymorphisms, protein levels and functional activity. Mol. Immunol. 92: 125-131 (2017)
Varade, J., Wang, N., Lim, C.K., Zhang, T., Zhang, Y., Liu, X., Piehl, F., Matell, R., Cao, H., Xu, X., Hammarström, L. Novel genetic loci associated HLA-B-08:01 positive myasthenia gravis. J. Autoimmun. 88: 43-49 (2018)
Weinstock, C., Matheis, N., Barkia, S., Haager, M.C., Janson, A., Markovic, A., Bux, J., Kahaly, G.J. Autoimmune polyglandular syndrome type 2 shows the same HLA class II pattern as type 1 diabetes. Tissue Antigens 77: 317-324 (2011)
Wieczorek, S., Knaup, S., Gross, W.L., Epplen, J.T. Genetic variability of RXRB, PPARA, and PPARG in Wegener's granulomatosis. PPAR Res. 2009: 786781 (2009)
Wiencke, K., Spurkland, A., Schrumpf, E., Boberg, K.M. Primary sclerosing cholangitis is associated to an extended B8-DR3 haplotype including particular MICA and MICB alleles. Hepatology 34: 625-630 (2001)
Yeo, T.W., De Jager, P.L., Gregory, S.G., Barcellos, L.F., et al. A second major histocompatibility complex susceptibility locus for multiple sclerosis. Ann. Neurol. 61: 228-236 (2007)
Yousefipour, G.A., Salami, Z., Farjadian, S. Association of HLA-DQA1-0101/2 and DQB1-0502 with myasthenia gravis in southern Iranian patients. Iran J. Immunol. 6: 99-102 (2009)

Good lecture on immunology. Maybe this will help me convince my grandkids they should eat some dirt, play with farm animals, and at least swim in a creek instead of taking excessive antibiotics for everything and an antihistamine for every sneeze!
I had the 23andMe genetic analysis performed for myself and it came out pretty good overall. The only real bad news was that my probability for getting rheumatoid arthritis was ~5x greater than the population average.
It seems there is nothing you can do to prevent this type of arthritis but when cannabis becomes legal here in Canada I intend to start taking CBD (the non-psychoactive component, I don't like getting stoned) as it apparently shows promise for inflammatory diseases.
I am looking forward to better research on these compounds in the future now that it is becoming a more acceptable research topic.
Promethease analysis of 23andMe file excerpt.
I think that rs6679677 is a polymorphism associated with or near the PTPN22 gene on chromosome 1 and is mentioned in these 4 articles:
https://www.ncbi.nlm.nih.gov/pubmed/22493691
https://www.ncbi.nlm.nih.gov/pubmed/20089178
https://www.ncbi.nlm.nih.gov/pubmed/20722033
https://www.ncbi.nlm.nih.gov/pubmed/18305142
This would be one of the 'secondary genetic risk factors' for autoimmune disease.
CBD oil has been reported to be effective in treating inflammation associated with arthritis (https://www.ncbi.nlm.nih.gov/pubmed/16282192) and so your plan is good!
The main genetic risk factor for rheumatoid arthritis is the HLA-DRB1 gene carrying the "shared epitope" (SE) allele(s). There appears to be an interaction between this HLA-DRB1 SE and the environmental risk factor, smoking. Together this promotes development of antibodies to citrullinated proteins (proteins that have arginine residues that have been modified to citrullines (an enzyme peptidyl arginine deiminase (PAD) catalyzes this reaction; PAD may be induced by smoking!)). Furthermore, polymorphisms in the PTPN22 gene further increase development of antibodies to citrullinated proteins (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1852748/). So if you carry an HLA-DRB1 SE allele, and you are a smoker, and you carry an R620W PTPN22 gene variant, odds ratios for developing rheumatoid arthritis go up substantially.
Major Histocompatibility Complex (MHC Class I and II)