Gender Differences in Autoimmune Diseases
Summary
It has been frequently recognized that autoimmune diseases are more common in females than in males (Zandman-Goddard et al. (2007), Voskuhl (2011), Nussinovitch and Shoenfeld (2012), Ngo et al. (2014)). Why this gender difference? Various hypotheses have been proposed to explain this sex bias, and these include:
- genes carried on the sex chromosomes (X and Y)
- sex hormones
- gut microbiome
- exposure to different environmental factors in males versus females
In this article I will attempt to address these 4 hypotheses which shed light on the gender influence on autoimmunity.
Gender definition
At the outset I would like to emphasize that I will be using the definition of "gender" employed in the vast majority of the medical literature, where "gender is synonymous with biologically determined sex" (Pollard (2012)). So, for the most part, I will be discussing biological differences between males and females. This is not meant to be disrespectful to Facebook users who can now choose from 71 genders: Facebook's 71 gender options come to UK users - The Telegraph.
A brief overview of the genetics of gender and biologically determined sex
So what are the genetic determinants of being male versus female? Most of you will know that in humans (and other mammals) one's biological sex is dependent upon (at conception) receipt of an X chromosome from the female parent and either an X chromosome or a Y chromosome from the male parent. If the resulting egg has two X chromosomes (XX) the child will be female. If the resulting egg has one X chromosome and one Y chromosome (XY) the child will be male. Sounds simple, but it is a more complicated than this! The Y chromosome carries a critical gene called the sex-determining region Y (SRY) protein, also known as the testis-determining factor (TDF), that primarily determines sex. This gene regulates the expression of a substantial array of genes that determine development of male sex organs. In the absence of SRY, female sex organs develop.
Occasionally (during meiosis) the SRY gene can be transferred to the X chromosome by crossover, in which case the resulting Y chromosome will not carry an SRY gene. Individuals carrying such a Y chromosome (lacking SRY) will have an apparently male karyotype, but will not undergo testis development and will have a female phenotype.
The X chromosome that results from a crossover event of the type described above will now have an SRY gene, and therefore the ability to initiate testis development. Offspring who inherit this X chromosome will have a condition called XX male syndrome, characterized by an XX karyotype, and a male phenotype.
Where the Y chromosome carries an SRY gene with a mutation that renders the gene inactive, this can also result in a female phenotype.
The normal (wildtype) SRY gene therefore determines testis (Barrionuevo et al. (2012)) and prostate differentiation (Meeks and Schaeffer (2011)) and promotes the biosynthesis of male hormones, androgens (mainly testosterone and dihydrotestosterone).
The androgen receptor is a protein encoded by an X-linked gene. The androgen receptor protein interacts with the SRY gene product and probably directs transcription of many genes that contribute to the male phenotype. Defects in the androgen receptor gene can lead to the syndrome of testicular feminization, or androgen insensitivity syndrome "an intersex condition in which there is a partial or complete inability of many cells in the affected genetic male to respond to androgenic hormones".
Interestingly, SRY regulates nervous system development (Turner et al. (2011)), brain differentiation (Rosenfeld (2017)) and dopamine levels. So yes, ladies, men do have different brains!
SRY also down-regulates the enzyme aromatase that converts testosterone to the female hormone estradiol (the major estrogen sex hormone) (Ghosh et al. (2018), Spinello et al. (2018), Cisternas et al. (2018)). Thus, in the absence of a functional SRY gene, estrogen production is accelerated at the expense of testosterone: i.e. male hormone is converted to female hormone.
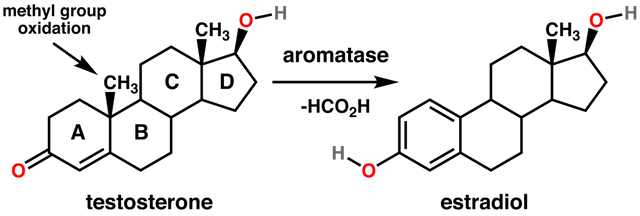
General reaction for the conversion of testosterone to estradiol catalyzed by aromatase. Steroids are composed of four fused rings (labeled A-D). Aromatase converts the ring labeled "A" into an aromatic state. Image Source:
For more details on the genetics and physiology of sex determination in humans and related species, please consult the following references: Haqq et al. (1993), Haqq et al. (1998), McElreavey et al. (1995), Barrionuevo et al. (2012), Waters et al. (2007), Ditewig et al. (2005), Ely et al. (2010), and Manolakou et al. (2006).
X-inactivation
To complicate matters, in female mammals (including humans) one of the X chromosomes is inactivated "by it being packaged in such a way that it has a transcriptionally inactive structure called heterochromatin. As nearly all female mammals have two X chromosomes, X-inactivation prevents them from having twice as many X chromosome gene products as males." (see X-inactivation). This must occur in every cell of the female's body. Most often the paternal X is inactivated as frequently as the maternal X chromosome, i.e. it is random. Thus, most females are essentially chimeras of cells expressing either their father's X chromosome, or expressing their mother's X chromosome. Occasionally there is skewed X-inactivation in which one parental X chromosome is favored over the other, leading to an uneven number of cells with each chromosome inactivated. Extreme skewing is when over 90% of cells have inactivated the same X chromosome (either paternal or maternal). For further details of the X-inactivation machinery and its regulation please see Ahn and Lee (2010).
Notably the X chromosome is gene-rich, while the Y chromosome is gene poor (see Genes on human chromosome X and Genes on human chromosome Y). The X chromosome carries a number of genes which are critical for normal immune response (FOXP3 is a notable example; FOXP3 is a master regulator of regulatory T cells which suppress autoimmunity). Skewed X-inactivation could potentially expose specific paternal or maternal X-linked alleles that are defective and which may predispose to certain diseases such as autoimmune disease.
Gender ratios in autoimmune diseases
The following is a list of gender ratios reported for a variety of autoimmune diseases derived from Pollard (2012), Eder et al. (2012), Herzog et al. (2014), Liang et al. (2017), Ngo et al. (2014), Megiorni and Pizzuti (2012), and Weismüller et al. (2017). Links to Wikipedia articles giving background information on each autoimmune disease are provided:
Autoimmune Disease / Ratio (female/male) / Reference(s)
- Sjögren syndrome 9:1 - 20:1 (Pollard (2012))
- Primary biliary cirrhosis/cholangitis 9:1 - 10:1 (Pollard (2012))
- Systemic lupus erythematosus 9:1 (Pollard (2012))
- Antiphospholipid syndrome 9:1 (Pollard (2012))
- Mixed connective tissue disease 8:1 (Pollard (2012))
- Autoimmune hepatitis 8:1 (Pollard (2012))
- Graves' disease 8:1 (Pollard (2012))
- Hashimoto's thyroiditis 8:1 (Pollard (2012))
- Systemic sclerosis (scleroderma) 3:1 - 5:1 (Pollard (2012))
- Rheumatoid arthritis 3:1 - 4:1 (Pollard (2012))
- Myasthenia gravis 2:1 - 3:1 (Pollard (2012))
- Multiple sclerosis 2:1 - 3:1 (Pollard (2012))
- Autoimmune thrombocytopenic purpura 2:1 (Pollard (2012))
- Diabetes mellitus type 1 1:1 - 2:1 (Pollard (2012))
- Celiac disease 1:1 - 2:1 (Ngo et al. (2014), Megiorni and Pizzuti (2012))
- Psoriasis 1:1 (Eder et al. (2012))
- Psoriatic arthritis 1:1 (Eder et al. (2012))
- Crohn's disease 1:1 (Ngo et al. (2014))
- Ulcerative colitis 1:1 (Pollard (2012))
- Autoimmune myocarditis 1:1.2 (Pollard (2012))
- Primary sclerosing cholangitis 1:2 (Liang et al. (2017), Weismüller et al. (2017)
- Crohn's disease in childhood 1:2.6 (Herzog et al. (2014))
- Ankylosing spondylitis 1:3 - 1:5 (Eder et al. (2012))
Some autoimmune diseases (e.g. Sjögren’s syndrome, primary biliary cirrhosis (now known as primary biliary cholangitis) and systemic lupus erythematosus) show a striking female bias of over 9 (female) : 1 (male). Only a relatively few diseases (e.g. ulcerative colitis) appear to be gender neutral with a female : male ratio of 1:1. Peculiar exceptions (not discussed by Pollard (2012)) are primary sclerosing cholangitis which has a male bias and a female : male rato of 1:2 (Liang et al. (2017), Weismüller et al. (2017)), and ankylosing spondylitis with a female : male ratio of 1:3 - 1:5 (Eder et al. (2012)).
Skewed X-inactivation and autoimmune disease
Skewed X-inactivation has been observed to be associated with several autoimmune diseases that have a strong female bias, including autoimmune thyroid diseases (Graves' disease and Hashimoto's thyroiditis) (Brix et al. (2005), Ozcelik et al. (2006), Yin et al. (2007), Simmonds et al. (2014)), rheumatoid arthritis (Chabchoub et al. (2009)), and systemic sclerosis (Broen et al. (2010)). Broen et al. (2010) demonstrate that skewed X-inactivation in systemic sclerosis is associated with reduced expression of the X-linked gene, FOXP3, and reduced suppressive capacity of regulatory T cells. One of the main functions of regulatory T cells is to suppress pro-inflammtory Th17 cells (Maddur et al. (2012) ... and this subject must be deferred to a future article due to its complexity.
GPR173 has recently been identified as an X-linked gene that may play a role in the female bias for systemic lupus erythematous (Zhang et al. (2018)).
DNA methylation
It has recently been shown that there is sexual dimorphism in DNA methylation resulting from an interaction between a Y-linked factor (SRY) and at least one X-linked factor that acts in a dose-dependent manner (Ho et al. (2018)). It is suggested that the resulting difference in methylation of specific genes between males and females may predispose females to autoimmune disorders (Ho et al. (2018)).
Sex hormones and autoimmune disease
As described by Yurkovetskiy et al. (2013) this sexual dimorphism (where females have a higher incidence and/or severity of autoimmune disease than males) may be primarily the result of sex hormones, with androgens being protective. Thus, castration of male animals renders them more susceptible to disease, and supplementation of females with androgens affords protection against autoimmune diseases with a strong female bias, such as systemic lupus erythematosus and type 1 diabetes. However, the role of sex hormones in autoimmune diseases with a male bias (e.g. primary sclerosing cholangitis and ankylosing spondylitis) has received comparatively little attention. Sex hormones have a profound effect on glucocorticoid receptor signaling and inflammatory gene expression (Fairweather et al. (2008), Duma et al. (2010), Quinn et al. (2016)).
Gender and smoking interactions
Whereas many of the above autoimmune diseases are promoted by smoking (Pollard (2012)), surprisingly in ulcerative colitis and primary sclerosing cholangitis (diseases with gender neutrality or a male bias, respectively) smoking is protective against disease development (Birrenbach and Böcker (2004), Calkins (1989), Liang et al. (2017), Loftus et al. (1996), van Erpecum et al. (1996)), suggesting that the smoking : gender interaction deserves further investigation. Because smoking has been recently shown to influence the intestinal microbiome (Savin et al. (2018)) and there is growing evidence that the microbiome may have a profound effect on development of autoimmune disease in a gender-specific manner (see below), the smoking : gender : microbiome interactions are also of growing interest.
Gender and the microbiome
Yurkovetskiy et al. (2013) showed that in a mouse model of type 1 diabetes (nonobese diabetic (NOD) mice) females are more prone to disease development, but when these mice are rendered germ-free, the gender bias disappears. The authors also showed that the gut microbiota differed between males and females and that this trend was reversed by castration. Moreover, supplementation with androgen supported expansion of protective microbial lineages, suggesting a "two-signal model of gender bias in which hormones and microbes together trigger protective pathways" (Yurkovetskiy et al. (2013)). This model has also been investigated by Markle et al. (2013) where transfer of gut microbiota from adult males to immature females resulted in elevated testosterone levels, reduced inflammation and autoantibody production, and protection against development of type 1 diabetes. Here the authors also demonstrated that these effects were critically dependent upon androgen receptor activity (Markle et al. (2013); see also commentaries by Leavy (2013) and Flak et al. (2013)). For a recent review of the role of the gut microbiome in autoimmunity and the importance of sex in modulating this interaction, please see Gomez et al. (2015).
Gender and exposure to xenobiotics
Exposure to various xenobiotics has been suggested to be a contributing factor to development of autoimmune diseases in women. Particular attention has been focused on cosmetics (Tanaka et al. (2018)) and hair dyes (Smyk et al. (2013)) that are more frequently used by women than men. The case of primary biliary cirrhosis/cholangitis is quite interesting as this liver disease that mostly affects women can be triggered by 2-octynamide, a conjugate derived from 2-octynoic acid present in cosmetics and lipsticks (Tanaka et al. (2018)).
References
Haqq, C.M., Donahoe, P.K. Regulation of sexual dimorphism in mammals. Physiol. Rev. 78: 1-33 (1998)
Voskuhl, R. Sex differences in autoimmune diseases. Biol. Sex Differ. 2: 1 (2011)
Previous article in this series
Interesting topic and very well written. I enjoyed it and learned from it.
Thank you. It took quite a bit of reading for me to put this together and I learned a lot in the process.
Hi @davidrhodes124!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Thank you!
Hi, we have voted on your post because you have posted your article to either food, recipe, recipes, cooking or steemkitchen #tag. Steemkitchen is a brand new initiative where we want to build a community/guild focused purely on the foodie followers and lovers of the steem blockchain. Steemkitchen is out of the conceptual phase and growing each day. We would love to hear your thoughts and ideas.
We are almost ready to Launch the first Decentralized Recipe and Food Blog Website that will utilize the Steem BlockChain and its community to reward contributions by its members.
Please consider joining us at our new discord server https://discord.gg/XE5fYnk
Also please consider joining our curation trail on https://steemauto.com/ to help support each other in this community of food and recipe lovers.
Kind Regards
@steemkitchen
Ps. Please reply “No Vote” if you prefer not to receive this vote and comment in the future.
I do not have any blood relation with you, but the relation between humanity is to help my brother for humanity
Sir plz my one request

This is not my steem I'd this is my brother I'd his is name is haseebkhan
My brother haseebkhan is so I'll he is in hospital his one kidny is damag 1.70 cm 10 Stones his kidney and very pin full plz help my brother and doctor advised oprishan and pay 5000 US dollars
Plz sir I request you help my brother and save my brother life plz
Check report