MEDICAL PHYSICS: Therapy Using Ionizing Radiations, Laser Treatment and the Production of Artificial isotopes.
This is a continuation from where I stopped in
my last post on MEDICAL PHYSICS: Imaging using Light, Radioactive Tracers and Therapy using Ultrasound, discussing about therapy using ionizing radiations. In this article, I will also explain the production of
artificial isotopes and therapy using laser treatment.
In some cases, the therapy using ionizing radiations require more energetic photons, and these are actually ‘artificial’ gamma rays, produced in supervoltage devices – linear or circular electron accelerators. These instruments accelerate electrons using potential differences between 4 MV and 42 MV, and the electrons give rise to the high-energy photons.
Teletherapy uses high-energy gamma rays from radioactive nuclides. (‘Tele’ means ‘at a distance’.) These gamma rays bridge the gap between X-rays and photons from supervoltage devices. The nuclides commonly used are caesium-137 and cobalt-60. Both have long enough half-lives for the output from the source to be roughly constant during a course of treatment. The source is placed in a lead-lined steel container which emits the gamma rays through a small aperture. The source has the advantage of being small, and simpler to use than X-ray machines. However, it cannot be turned off and is a small but significant radiation hazard for the medical staff working with it.
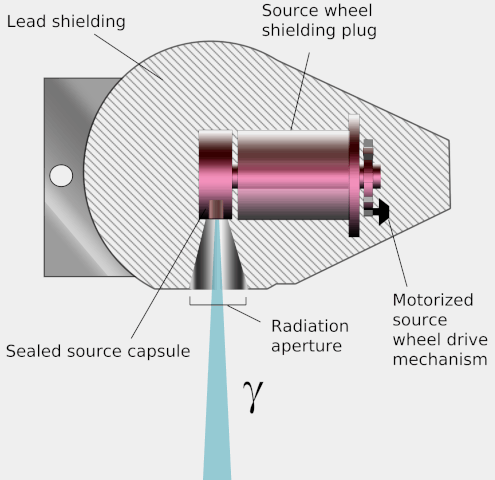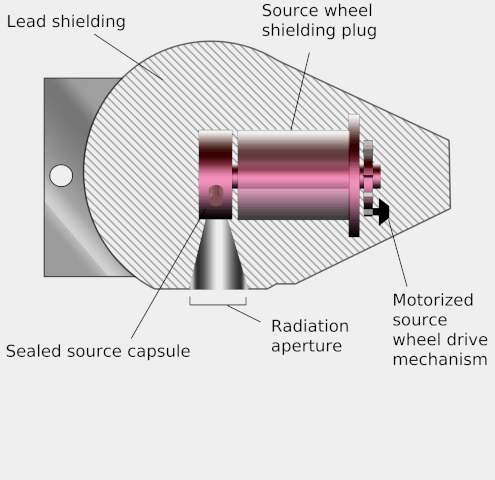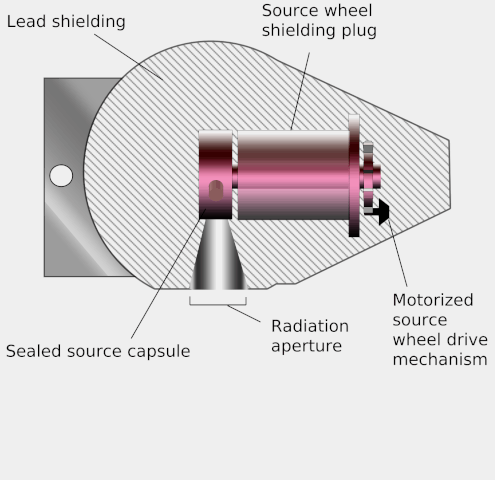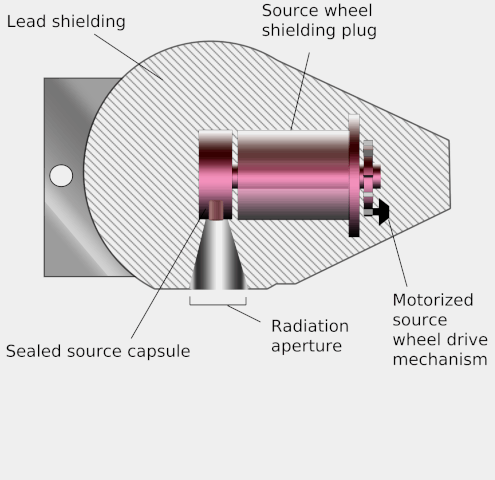
Diagram of a teletherapy device showing its operation. Wikimedia, KDS4444, public domain
Implanted radiation sources are also used to treat Malignant tumours. These deliver a small but continuous dose of radiation at the site of the tumour, which kills the cells. ‘Needles’ contain a radionuclide (such as radium-226 or gold-198). The use of small sources can produce very localized effects. This is especially important with radium which is an alpha emitter.
Unsealed sources can be injected or ingested (swallowed), and are used when they accumulate selectively in malignant tissue. For example, radioactive iodine accumulates in the thyroid gland and so is used to treat cancer of that gland. Colloidal suspensions of gold-198 target malignant cells carried in fluids lining the lungs and in the abdominal cavity.
The production of artificial isotopes
Most of the radioactive materials used in medicine do not occur naturally but are produced in nuclear fission reactors at nuclear power stations from natural, often stable, isotopes. Usually, a sample of the natural isotope is irradiated with neutrons obtained as a by-product in the reactors.
One reaction involves the capture of a neutron by the nucleus, which then immediately emits a gamma ray and also becomes a radioactive isotope of the original nuclide. This is the n,γ reaction. For example: ordinary sodium-23 is converted to radioactive sodium-24:
2312Na + 10n → 2411Na + γ
In this process the element stays the same. Not all the nuclei will be changed. It is chemically impossible to separate the two isotopes, so that you cannot get a pure sample of the active isotope. Ordinary sodium is the carrier for radioactive sodium. Phosphorus-32 and potassium-42 are other examples of tracers that can be produced by neutron irradiation.
A pure sample of the radioactive isotope phosphorus-32 can be made using another reaction: ordinary (stable) sulphur is irradiated by neutrons and the irradiated nuclei emit protons in the n,p reaction:
3216S + 10n → 3215P + 11p
The new nuclide is chemically different from the irradiated nuclide, and so it can be separated chemically. This process is also used to produce carbon-14 from nitrogen-14, and sulphur-35 from chlorine-35. These pure and entirely radioactive substances are called carrier-free.
Other processes include the n,α reaction, which also produces pure samples. Phosphorus-32 is an example:
3517Cl + 10n → 3215P + 42α
The phosphorus is then separated chemically from the chlorine. This process is also used to produce hydrogen-3 (tritium) from lithium-6.
The very useful tracer isotope metastable technetium-99m is produced when the radioactive isotope of molybdenum, 9942Mo, decays by beta emission:
9942Mo + 99m43Tc → 3215P + 0-1e + ṽ
(Inside the nucleus, n → p + e- + ṽ.)
This process has a half-life of 67 hours, and the technetium can be produced using ‘molybdenum-cow’, a column of alumina into which the molybdenum has been absorbed. When required, the technetium can be flushed out with a saline solution, leaving the insoluble molybdenum behind, although the solution must then be cleaned of aluminium and other impurities.

The discovery of neon isotopes. Wikimedia, Public Domain
PHYSICAL, BIOLOGICAL AND EFFECTIVE HALF-LIFE
The activity of a radioactive isotope decreases with time with an exponential decay signified by its physical half-life. Here I will denote this by tr. But when the isotope is in the body it effectively gets weaker because it is usually removed from the body by biological routes: the waste removal processes of respiration, urination and defecation. By how much each of these processes decreases effective activity depends upon the chemistry of the isotope and the part of the body in which it is used. For example, iodine-131 is used to label the human protein serum albumin. I-131 has a physical half-life of 8 days, but it is removed from the body with a half-life of 21 days; this is its biological half-life, tb. The effective half-life, te, is a combination of the two:
1/t = 1/tr + 1/tb
EXAMPLE
What is the effective half-life of technetium-99m in the body when its biological half-life is 15 hours? The half-life of Tc-99m is 6 hours.
Answer: Inserting the data into the formula above gives
1/te = 1/6 + 1/15
=(5+2)/30
So, te = 30/7 = 4.3 hours
DILUTION ANALYSIS
How much plasma does the patient’s blood contain?
Plasma is the colourless liquid in the blood that carries – amongst other things – the blood cells that make blood red. Plasma contains proteins which can be labelled with iodine Isotopes such as I-131, at the rate of about one atom of the isotope per protein molecule in a sample of plasma. The most common method is to label the protein serum albumin with I-131, which has a half-life of 8 days and emits gamma rays. Labelled serum albumin in a volume v of distilled water is injected into the blood stream. A sample of equal activity is diluted with a known volume of distilled water and kept as a comparison standard. Fifteen minutes later a blood sample is taken and centrifuged to separate out the plasma.
The activity of this sample is compared with that of an equal volume (the comparison sample) in a scintillation counter. The gamma photons collide with atoms and energize them. As the electrons fall back to the lower state they emit light photons. These photons are few and are detected by using a photomultiplier tube.
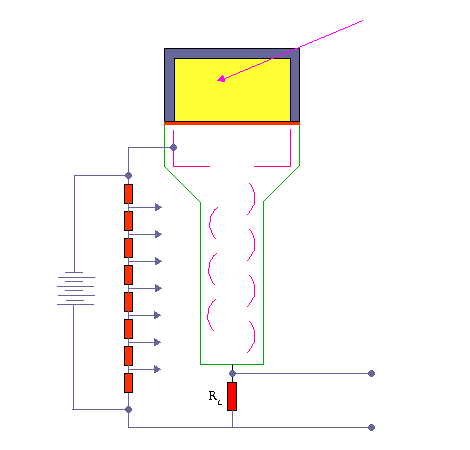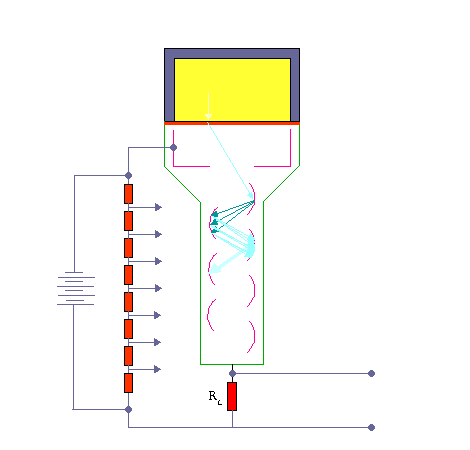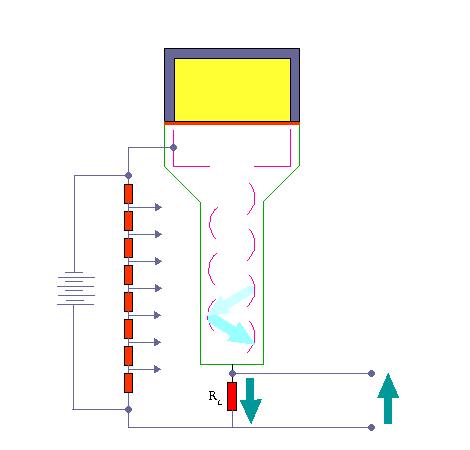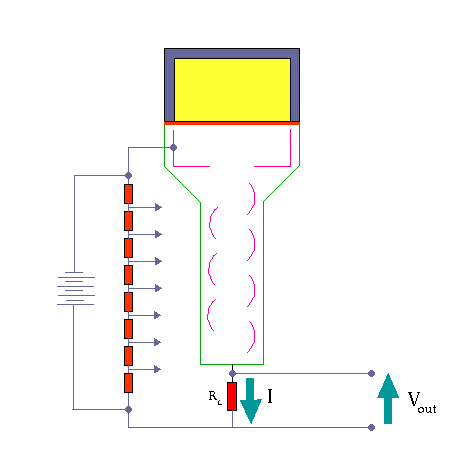
Scintillation Detector. KieranMaher at English Wikibooks, Public Domain
Calculating the result
Suppose the sample from the body has an activity p, and the standard sample an activity s. The value of p will be less than s because the injected sample has been diluted by the volume of plasma in the patient’s body, whilst the standard sample has been diluted by a much smaller known volume of water.
A volume v of tracer is injected into a body with plasma volume V.
Suppose the original activity of the tracer was A. Then for the body sample, this is reduced by a factor v/V, so P = A.v/V.
For the reference sample, the activity is reduced by a factor d. So, s = A/d.
They both started off with the same activity and both have been reduced by an equal amount due to normal decay. Thus it is fair to compare the activities bearing in mind just the dilution effects. A just cancels so we have:
s/p = 1/d ÷ v/V
And, as the original activities were the same:
s/p = V/vd
and:
V = vds/p
In practice, a number of corrections have to be made to allow for such things as background radiation. An average man of body mass 70 kg has a blood plasma volume of about 3 litres.
THERAPY USING LASER TREATMENT
Laser light carries energy and can be focused into a small volume. This energy can destroy living tissue. Malignant tissue (for instance, a cancer tumour) absorbs laser radiation more strongly than healthy tissue: the output from lasers is usually pulsed and each pulse contains a definite quantity of energy. The wavelength of radiation, its intensity and the time of exposure are chosen to suit the absorption properties of the tumour.
In the eye, a detached retina can be spot-welded – coagulated – back on to the wall of the eyeball by laser light.
A laser can produce light with enough energy to cut through tissue. As it does so, it ‘heat seals’ blood vessels and so there is less bleeding than during scalpel surgery. With this technique, diseased parts of the liver can be removed, whereas using a knife might lead to a life-threatening loss of blood.
Darkly pigmented tissues absorb best and this is why lasers are also used to decolour skin blemishes, such as ‘port wine’ birth-marks.
MONITORING RADIATION DOSE OF HEALTH WORKERS
Medical workers and patients are protected as far as possible from unnecessary exposure to ionizing radiation, by careful monitoring of the radiation dose they receive.

Dosimeter. Ditlev Petersen - Own work,CC BY-SA 3.0
One of the most common monitoring device for medical workers is the film badge. The film badge contains two types of film: one is fast (sensitive), the other slow (less sensitive). The films are in a light-tight box and radiation enters through three windows. One is ‘open’: the light-tight cover does not include an absorber and lets all the radiation through. The other two areas are covered with several absorbing filters which have two functions: to indicate the degree (total exposure) of irradiation, and to identify the type of radiation:
- Irradiated film shows darkening when it is developed, and the image density depends on the total exposure to radiation.
- Low-energy radiation darkens the part of the film with no absorber or a low absorber in the way, but has little effect in other parts. The higher the energy of the photons and particles, the more likely they are to pass through the filters of increasing thickness or density, and so the more likely they are to reach and affect the underlying film. Blank areas show that no high-energy radiation has been received. In general, the contrast between the different areas of the film indicates the range of radiations the user has been exposed to.
The filters and film speeds are chosen to simplify analysis of the type and degree of exposure. The badges are cheap, reliable and sufficiently accurate for their monitoring task, measuring exposure dosage to about 20 per cent.
SUMMARY
After going through all my articles on medical physics, you should have understood and been able to describe the following main topics and techniques related to medical physics:
- How non-invasive techniques are important for diagnosis and treatment.
- That ultrasound is generated and detected by piezolectric crystals, and is used to produce images.
- How ultrasound is used for diagnosis, body scanning and blood flow measurements; there is a relationship between the frequency used and the resolution obtained.
- How X-ray beams of different types are generated and detected to produce images.
- That X-rays interact with matter: they become filtered and attenuated, and so may be controlled to produce images (shadowgraphs).
- That X-rays for treatment are generated by high voltages. X-rays reach the patient as multiple beams or as a single beam in rotational treatment.
- How magnetic resonance imaging is used as a non-hazardous imaging technique.
- How ingested and injected radioisotopes are used for body measurements, diagnosis and therapy.
- How medically useful radioisotopes are produced (e.g. technetium-99m) from naturally occurring isotopes.
- How the following instruments are used to detect and monitor ionising radiations and to make Images: film, Geiger and scintillation counters and photomultiplier tubes.
- What safety aspects are involved in the use of ultrasound and ionizing radiations.
Till next time, I still remain my humble self, @emperorhassy.
REFERENCES
https://www.ncbi.nlm.nih.gov/books/NBK232715/
https://opentextbc.ca/physicstestbook2/chapter/therapeutic-uses-of-ionizing-radiation/
https://www.iaea.org/topics/radioisotope-production-in-research-reactors
https://glossary.periodni.com/glossary.php?en=artificial+radioactive+isotope
https://en.wikipedia.org/wiki/Synthetic_radioisotope
https://radiopaedia.org/articles/half-life-time-1
https://nucmedtutorials.files.wordpress.com/2016/12/half-lives1.pdf
https://www.labcorp.com/resrouce/blood-specimens-chemistry-and-hematology
https://en.wikipedia.org/wiki/Blood_volume
http://tech.snmjournals.org/content/35/2/55.full
https://en.wikipedia.org/wiki/Low-level_laser_therapy
http://www.electrotherapy.org/modality/laser-therapy
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Hi @emperorhassy!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV