MOLECULES IN INTERSTELLAR SPACE AND A CLOSE LOOK AT ELECTRONS #4
Hello, great readers. This is the concluding part of my article on MOLECULES IN INTERSTELLAR SPACE AND A CLOSE LOOK AT ELECTRONS. It is a promise to @chappertron as regards his request that I give a summary of the entire article on behalf of @steemstem. But, I felt rather than writing a summary, why don’t I give him the honour and respect of writing an entirely comprehensive article on the request. Also using the opportunity to discuss the electron configuration and its relationship to the Periodic table. I hope you all find it interesting.
So, why don’t I pick up, specifically, from where I stopped in the previous article, by shedding more light on the evidence for subshells from the first ionization energies?

The shapes of the first five atomic orbitals. Mortadelo2005, Public Domain
THE EVIDENCE FOR SUBSHELLS FROM FIRST IONIZATION ENERGIES
The evidence for subshells also comes from ionization energies. If we plot the first ionization energy of different elements against their atomic numbers, a regular pattern emerges. The repeating pattern of a property is called periodicity. It forms the basis of the Periodic Table, which I’ve written extensively about here.
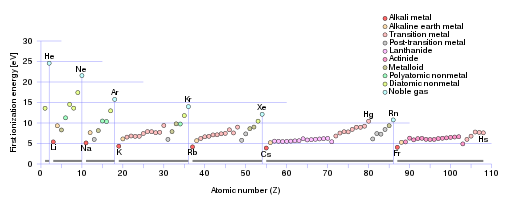
Several features of the graph point to the existence of subshells. let’s look at the eight elements lithium to neon, which have electrons in shell 2. We would expect an increase in the first ionization energy as the atomic number increases. This is because the number of protons in the nucleus increases, which increases the nuclear charge. If the electrons were all in the same energy level, we would expect the graph to be a straight line, showing a steady increase in the first ionization energy as the number of protons in the nucleus increases.
The 2,3,3 pattern of first ionization energies tells us that the electrons removed from this second electron shell are not arranged such that they are all in the same energy level.
From reading this section on subshells, you may be able to explain the 2,3,3 pattern. Refer to the figure as you read on.
The first part of the 2,3,3 pattern is caused by electrons being taken from the 2s subshell. There is an increase in first ionization energy from Li to Be because Be has an extra proton attracting the outer electrons. Then, instead of a further increase from Be to B, there is a decrease in first ionization energy as the electron in B is taken from the 2p subshell: this electron is at a higher energy level than the 2s subshell and further from the nucleus.
In moving from B to N. There is the expected increase in first ionization energy, but another dip occurs at O. After N. The electrons in the 2p subshell start to pair up. (This pairing of electrons in subshells is covered in some paragraphs below.) There is more electrostatic repulsion between paired electrons, which means that the fourth electron in the 2p subshell is easier to remove, explaining the dip at O.
You can see the same pattern repeated for elements starting with Na when the next shell is being filled. After Mg, there is a drop at Al, and another drop at S. The 3p subshell is complete at Ar.
After this, there is an interruption to the 2,3,3 pattern. There is the expected increase from K to Ca as electrons are taken from the same 4s subshell. Then we have electrons removed from the slightly higher energy 3d subshell before the 3,3 pattern returns, beginning with Ga, as the 4p subshell electrons are removed.
ATOMIC ORBITALS
Another great scientist of the 20th century was Werner Heisenberg, whose work has clarified our current model of the way electrons are arranged in atoms. In 1927, he said that you could determine either the speed of an electron or its position, but not both at the same time. This is now known as the Heisenberg uncertainty principle. While it applies to any particle, it becomes important only when the particle is very tiny. Heisenberg’s mathematics has enabled electron arrangements to be worked out in even more detail.
We have seen that lines appear in the emission spectra of excited atoms, representing the wavelengths (energies) of photons emitted by excited electrons returning to lower energy levels. When this light is passed through a magnetic field, even more lines show up, and these are evidence of what we can now call atomic orbitals.
An atomic orbital is a region around the atom in which there is a high probability of finding an electron at any moment in time.
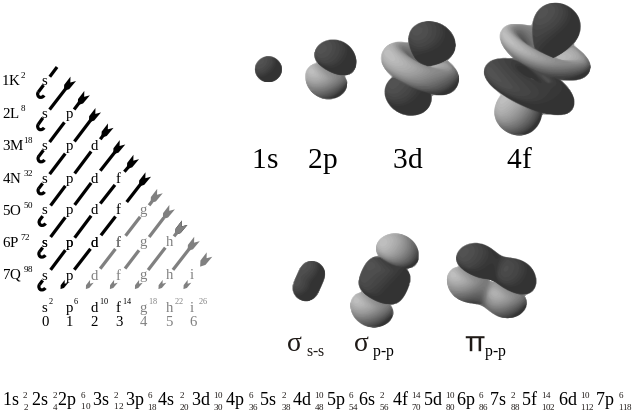
Advanced mathematics can be used to plot the probable position of an electron at one instant, followed by further plots at other instants until an electron density plot or electron density map is produced. The existence of electrons in orbitals helps the chemistry of atoms and molecules to fall into place, and so is tremendously useful. In the s subshell there is only one orbital, the s orbital. If we draw around the region of the atom where the electron spends most of its time, then we find the s orbital is spherical.
In the boundary surface of 1s, 2s and 3s orbitals, in each case, the imaginary ‘surface’ is the limit of the space in which the 1s, 2s and 3s electrons are likely to be found. Also in each case the orbital is centred on the nucleus, and the boundary represents a 90 per cent probability of finding an electron within it.
There are three p orbitals in the p subshell. They are dumb-bell shaped and arranged at right angles to each other, with the nucleus in the centre of each dumb-bell.
The d subshell contains five d orbitals, but there are seven f orbitals in the f subshell.
SPINNING ELECTRONS
Each atomic orbital can hold a maximum of two electrons. As the electrons move in their orbitals, they are also spinning on their own axes. If there are two electrons, one spins clockwise, while the other spins anticlockwise. This keeps the repulsion of the electrons to a minimum. Electron spin is often represented by box diagrams, in which each box is an atomic orbital and arrows do represent the electrons spinning in opposite directions as shown below.
Electron orbitals. Patricia.fidi - own work, Public Domain
ELECTRON ADDRESSES
So we are now in a position to give addresses to electrons. In the same way that a letter can be addressed with the country, the town. The street and the house number so that it arrives at only one unique destination, so we can give a unique ‘address’ to represent the state of an electron in an atom. The idea of unique state for every electron is known as the Pauli exclusion principle.
According to this principle, an electron is precisely defined by:
- its shell – the main energy level;
- its subshell – the sub-energy level division of the shell:
- its atomic orbital – each subshell possesses at least one atomic orbital, and all orbitals in a subshell possess the same energy;
- and its direction of spin – a maximum of two electrons of opposite spin can occupy one atomic orbital: orbitals in the same subshell are singly filled first, with electrons of parallel spin.
In the electron configuration of the magnesium atom in its ground state (I would have loved to share the image but it's copyrighted). Remember, this is the lowest energy state of the atom, so the electrons occupy orbitals in subshells of the lowest possible energy.
Notice that:
- the s subshells contain one s atomic orbital;
- the p subshells contain three p orbitals;
- each orbital contains a maximum of two electrons with opposite spin.
THE ELECTRON CONFIGURATIONS OF SOME ELEMENTS
Let us now look at how electrons fill orbitals in an atom. The orbitals of lowest energy are occupied first, and this is known as the aufbau principle. Aufbau is a German word for building up. So when you are building up the electron configuration of an element, remember that each electron goes into the lowest energy orbital available.
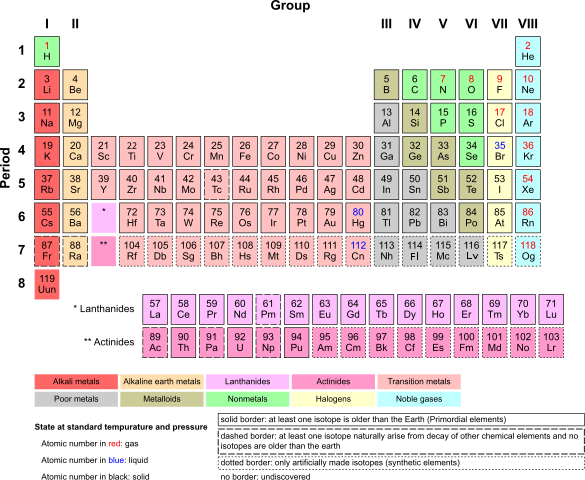
The Periodic Table. Armtuk, CC BY-SA 3.0
Hydrogen (Z = 1)
The lowest energy orbital is in the first shell that has a principal quantum number n = 1 and is an s orbital, so it is written 1s.
electron configuration 1s1
Helium (Z = 2)
electron configuration 1s2
Note: when you say 1s2, it is ‘one s two’, not ‘one s squared’!
Lithium (Z = 3)
electron configuration 1s2 2s1
The lithium atom has three electrons, and the aufbau principle tells us that the third electron will occupy the next orbital of lowest energy, which is in the n = 2 shell, so it will start with 2 and be an s orbital, and therefore it is 2s.
Beryllium (Z = 4)
electron configuration 1s2 2s2
Next, we come to filling the p subshell. Notice that each orbital is occupied singly at first. This is because two electrons in the same orbital exert a repulsion that raises the energy of a doubly filled orbital above that of a singly filled one. Notice that the spins are all in the same direction. The repulsion that results helps to keep the electrons apart.
Boron (Z = 5)
electron configuration 1s2 2s1 2p1
Carbon (Z = 6)
electron configuration 1s2 2s2 2p2
Nitrogen (Z = 7)
electron configuration 1s2 2s2 2p3
The next eleven elements follow the same pattern, filling up the s and p orbitals of the third shell.
Potassium and calcium come next. The 3d subshell is available, but remember that the 4s subshell has a lower energy, so it is filled first i.e
Potassium (Z = 19 )
electron configuration 1s2 2s2 2p6 3s2 3p6 4s1
Notice that we do not write 3d0 in the electron configuration. We leave it out.
Similarly, we can draw the box diagram for calcium like this:
Calcium (Z = 20)
electron configuration 1s2 2s2 2p6 3s2 3p6 4s2
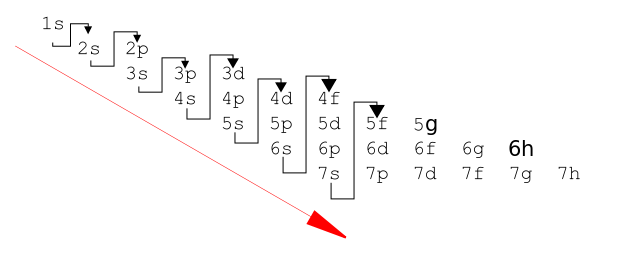
FILLING THE d ORBITALS (Z = 21-30)
The 4s Subshell has already been filled at calcium, so now the 3d subshell, which is at a slightly higher energy level, can begin to fill. Electrons spinning in the same direction first occupy the 3d orbitals singly, since this helps to minimize electron repulsion.
Notice that the chromium atom and the copper atom have only in the 4s atomic orbital.
GALLIUM (Z = 31) TO KRYPTON (Z = 36)
From gallium to krypton the 4p orbitals are filled because they have the next highest energy level
Gallium (Z = 31)
electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p1
Krypton (Z = 36)
electron configuration 1s2 2s2 2p6 3s2 3p6 3d10 4s2 4p6
ELECTRON CONFIGURATIONS AND THE PERIODIC TABLE
When we know the electron configurations of the elements, we can organize and explain the chemical properties of the elements, because it is the electron that determine these properties. Long before electrons were even known to exist, Mendeleev grouped together elements with similar properties in his Periodic Table. You can read much more about this in my previous article on THE HISTORY OF MENDELEEV AND THE DEVELOPMENT OF THE PERIODIC TABLE. After you might have gone through the article you will know about the Periodic Table, so let’s see how electron configurations fit in.
In Period 4, containing the transition elements, electron configurations show the two outermost subshells, because it is the d subshell that is being filled. Notice that the elements in each of the eight main groups end with the same number of electrons in their outer subshell. So Group 7 always has a p5 subshell and Group 2 always has a filled s2 orbital. The Periodic Table is often divided into blocks, as is shown in the diagram.
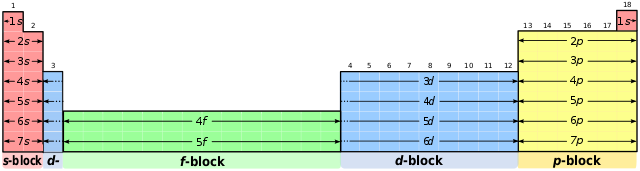
Electron configuration table. User:DePiep, CC BY-SA 3.0
So when we know how electrons are arranged in atoms, we have a better understanding of how the Periodic Table is arranged. It groups elements together with similar properties, and we can now see that it is the electron configuration that determines this similarity in properties.
SUMMARY
When you have gone extensively through all my posts on MOLECULES IN INTERSTELLAR SPACE AND A CLOSE LOOK AT ELECTRONS #1, #2, #3 and this final post, you should be able to comprehend the following ideas.
- Evidence for the arrangement of electrons in energy levels comes from the line emission spectra of atoms.
- Energy levels are quantized, which means that each level has a fixed amount of energy.
- An electron can move from a lower to a higher energy level by absorbing a quantum (packet) of energy. It releases the same quantum of energy when it falls back, as a photon of light of a particular frequency (which gives a line in the emission spectrum).
- The arrangement of electrons in an atom is called its electron configuration.
- Energy levels and sub-levels are also known as shells and subshells.
- Shells are numbered 1, 2, 3 and so on. The shell number is also known as the principal quantum number.
- The higher the shell number, the higher is its energy and the further the shell is from the nucleus.
- Successive ionization energies of atoms provide evidence of shells (energy levels).
- Within each shell there are subshells (sub-levels) s, p, d and f, of different energies. Shell 1 contains only an s subshell. Shell 2 contains two subshells, s and p, while shell 3 contains three subshells s, p and d.
- A plot of first ionization energy against atomic number provides evidence of subshells. In each subshell the electrons are arranged in atomic orbitals.
- Atomic orbitals are regions in the atom where there is a very high probability of finding the electron.
- There is one s atomic orbital in the s subshell, three p orbitals in the p subshell, five d orbitals in the d subshell and seven f orbitals in the f subshell.
- Each atomic orbital can accommodate two electrons of opposite spins.
- Finally, the Periodic Table is arranged in blocks, s, p, d and f, according to the subshell that is being filled.
PS: I made this article very deep, more detailed and extensive for the benefit of @steemstem courtesy of @chappertron. He is a great motivator and persuader. Lol
Thanks to y’all, for giving me the great opportunity.
REFERENCES
Ionization energy- chem.purdue
https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=25&cad=rja&uact=8&ved=2ahUKEwio4_yEtLHjAhVMRxUIHc67ClsQFjAYegQIBhAB&url=https%3A%2F%2Fwww.chemguide.co.uk%2Fatoms%2Fproperties%2Fatomorbs.html&usg=AOvVaw1RBKODGVZJpZspV_mrVwnh
https://en.wikipedia.org/wiki/Atomic_orbital
https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Electronic_Structure_of_Atoms_and_Molecules/Atomic_Orbitals
https://opentextbc.ca/chemistry/chapter/6-4-electronic-structure-of-atoms-electron-configurations/
https://en.wikipedia.org/wiki/Electron_configuration
https://www2.chemistry.msu.edu/faculty/reusch/VirtTxtJml/intro2.htm
Once again, a very nice and detailed post on this topic. This could be useful for anyone who would like to learn a little bit about it. Thanks for sharing!
Thanks for coming. I'm glad you like the post.
Very nice, thank you for this article. Now you can refer anytime to this nice written piece of information. Thanks & Respect
Chapper
Thank you, @chappertron. I really appreciate.
This post has been voted on by the SteemSTEM curation team and voting trail. It is elligible for support from @curie and @utopian-io.
If you appreciate the work we are doing, then consider supporting our witness stem.witness. Additional witness support to the curie witness and utopian-io witness would be appreciated as well.
For additional information please join us on the SteemSTEM discord and to get to know the rest of the community!
Thanks for having added @steemstem as a beneficiary to your post. This granted you a stronger support from SteemSTEM.
Thanks for having used the steemstem.io app. You got a stronger support!
Congratulations @empressteemah! You have completed the following achievement on the Steem blockchain and have been rewarded with new badge(s) :
You can view your badges on your Steem Board and compare to others on the Steem Ranking
If you no longer want to receive notifications, reply to this comment with the word
STOPVote for @Steemitboard as a witness to get one more award and increased upvotes!
Hi @empressteemah!
Your post was upvoted by Utopian.io in cooperation with @steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV