Ozone Depletion and its Effects
Good day steemit friends, yesterday i explained ozone and its formation, today i will be writing on ozone depletion, causes and its effect on Man and Agriculture.
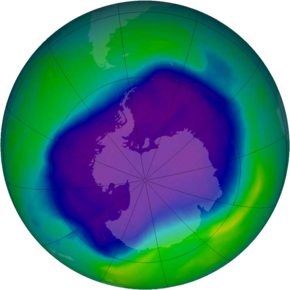
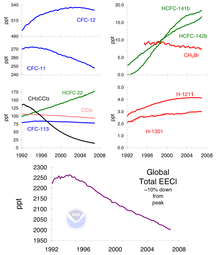
The main cause of ozone depletion and the ozone hole is man-made chemicals, especially man-made halocarbon refrigerants, solvents, propellants, and foam-blowing agents (chlorofluorocarbon (CFCs), HCFCs, halons), referred to as ozone-depleting substances (ODS). These compounds are transported into the stratosphere by the winds after being emitted at the surface. Once in the stratosphere, they release halogen atoms through photodissociation, which catalyze the breakdown of ozone (O3) into oxygen (O2). Both types of ozone depletion were observed to increase as emissions of halocarbons increased.
Ozone depletion and the ozone hole have generated worldwide concern over increased cancer risks and other negative effects. The ozone layer prevents most harmful UVB wavelengths of ultraviolet light (UV light) from passing through the Earth's atmosphere. These wavelengths cause skin cancer, sunburn, and cataracts, which were projected to increase dramatically as a result of thinning ozone, as well as harming plants and animals. These concerns led to the adoption of the Montreal Protocol in 1987, which bans the production of CFCs, halons, and other ozone-depleting chemicals.
The ban came into effect in 1989. Ozone levels stabilized by the mid-1990s and began to recover in the 2000s. Recovery is projected to continue over the next century, and the ozone hole is expected to reach pre-1980 levels by around 2075. The Montreal Protocol is considered the most successful international environmental agreement to date.
Ozone Cycle Overview
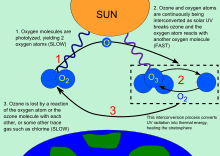
Three forms or allotropes of oxygen are involved in the ozone-oxygen cycle: oxygen atoms (O or atomic oxygen), oxygen gas (O2 or diatomic oxygen), and ozone gas (O3 or triatomic oxygen). Ozone is formed in the stratosphere when oxygen molecules photodissociate after intaking ultraviolet photons. This converts a single O2 into two atomic oxygen radicals. The atomic oxygen radicals then combine with separate O2 molecules to create two O3 molecules. These ozone molecules absorb ultraviolet (UV) light, following which ozone splits into a molecule of O2 and an oxygen atom. The oxygen atom then joins up with an oxygen molecule to regenerate ozone. This is a continuing process that terminates when an oxygen atom recombines with an ozone molecule to make two O2 molecules.
O + O3 → 2 O2
Ozone can be destroyed by a number of free radical catalysts; the most important are the hydroxyl radical (OH·), nitric oxide radical (NO·), chlorine radical (Cl·) and bromine radical (Br·). The dot is a notation to indicate that each species has an unpaired electron and is thus extremely reactive. All of these have both natural and man-made sources; at the present time, most of the OH· and NO· in the stratosphere is naturally occurring, but human activity has drastically increased the levels of chlorine and bromine. These elements are found in stable organic compounds, especially chlorofluorocarbons, which can travel to the stratosphere without being destroyed in the troposphere due to their low reactivity. Once in the stratosphere, the Cl and Br atoms are released from the parent compounds by the action of ultraviolet light, e.g.
CFCl3 + electromagnetic radiation → Cl. + .CFCl2
Ozone hole and its causes
As explained above, the primary cause of ozone depletion is the presence of chlorine-containing source gases (primarily CFCs and related halocarbons). In the presence of UV light, these gases dissociate, releasing chlorine atoms, which then go on to catalyze ozone destruction. The Cl-catalyzed ozone depletion can take place in the gas phase, but it is dramatically enhanced in the presence of polar stratospheric clouds (PSCs).
The role of sunlight in ozone depletion is the reason why the Antarctic ozone depletion is greatest during spring. During winter, even though PSCs are at their most abundant, there is no light over the pole to drive chemical reactions. During the spring, however, the sun comes out, providing energy to drive photochemical reactions and melt the polar stratospheric clouds, releasing considerable ClO, which drives the hole mechanism. Further warming temperatures near the end of spring break up the vortex around mid-December. As warm, ozone and NO2-rich air flows in from lower latitudes, the PSCs are destroyed, the enhanced ozone depletion process shuts down, and the ozone hole closes.
Most of the ozone that is destroyed is in the lower stratosphere, in contrast to the much smaller ozone depletion through homogeneous gas phase reactions, which occurs primarily in the upper stratosphere.
Effects
Effects on non-human animals
A November 2010 report by scientists at the Institute of Zoology in London found that whales off the coast of California have shown a sharp rise in sun damage, and these scientists fear that the thinning ozone layer is to blame. The study photographed and took skin biopsies from over 150 whales in the Gulf of California and found "widespread evidence of epidermal damage commonly associated with acute and severe sunburn", having cells that form when the DNA is damaged by UV radiation. The findings suggest "rising UV levels as a result of ozone depletion are to blame for the observed skin damage, in the same way that human skin cancer rates have been on the increase in recent decades.
Effects on crops
An increase of UV radiation would be expected to affect crops. A number of economically important species of plants, such as rice, depend on cyanobacteria residing on their roots for the retention of nitrogen. Cyanobacteria are sensitive to UV radiation and would be affected by its increase. Despite mechanisms to reduce or repair the effects of increased ultraviolet radiation, plants have a limited ability to adapt to increased levels of UVB, therefore plant growth can be directly affected by UVB radiation.
Increased tropospheric ozone
Increased surface UV leads to increased tropospheric ozone. Ground-level ozone is generally recognized to be a health risk, as ozone is toxic due to its strong oxidant properties. The risks are particularly high for young children, the elderly, and those with asthma or other respiratory difficulties. At this time, ozone at ground level is produced mainly by the action of UV radiation on combustion gases from vehicle exhausts.
Increased production of vitamin D
Vitamin D is produced in the skin by ultraviolet light. Thus, higher UVB exposure raises human vitamin D in those deficient in it. Recent research (primarily since the Montreal Protocol) shows that many humans have less than optimal vitamin D levels. In particular, in the U.S. population, the lowest quarter of vitamin D (<17.8 ng/ml) were found using information from the National Health and Nutrition Examination Survey to be associated with an increase in all-cause mortality in the general population.[50] While blood level of Vitamin D in excess of 100 ng/ml appear to raise blood calcium excessively and to be associated with higher mortality, the body has mechanisms that prevent sunlight from producing Vitamin D in excess of the body's requirements