Oxidation. The number one enemy of the metal elements
Oxidation comes from oxygen. And this word must be emphasized that it comes from Greek, specifically from the sum of two components of that language: "oxys", which can be translated as "acid", and "genos", which is equivalent to "produce".
Oxidation
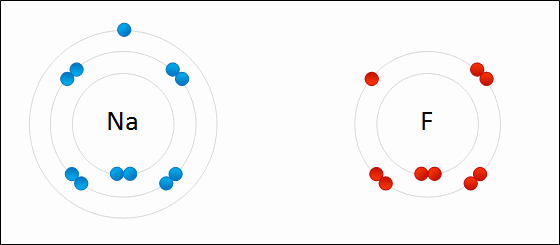
Oxidation is a chemical reaction where a metal or a non-metal yields electrons, and therefore increases its oxidation state. The chemical reaction opposed to oxidation is known as reduction, that is when a chemical species accepts electrons. These two reactions always occur together, that is, when a substance is oxidized, it is always by the action of another that is reduced. One gives up electrons and the other accepts. For this reason, the general term of redox reactions is preferred. Life itself is a redox phenomenon. Oxygen is the best oxidant that exists because the molecule is not very reactive (because of its double bond) and yet it is very electronegative, almost like fluorine.
However, the scientists saw that also other non-metallic elements of character, were combined with other substances in the same way that oxygen did. For example, oxygen burns in the presence of chlorine, but this also happens to antimony and sodium. Due to the similarity of the reactions, the scientists decided to expand the definition of oxidation, saying that oxidation was the process by which there was a loss of electrons in an atom, or in an ion. Thus, the substance participating in a reaction, which gains electrons, is known as an oxidizing agent, which will contain atoms that will be reduced in the reaction. If a substance, in a reaction, gains electrons easily, it will be said that it is a strong oxidizing agent.

Whenever oxidation occurs there is energy release
This energy can be released slowly, as is the case of oxidation or corrosion of metals, or it can be released very quickly and explosively as is the case of combustion. Oxidation is present everywhere and occurs in places we can not imagine. There are several types of oxidation, such as combustion, and some of them, as we can see, occur within the organism.
Respiration, one of the types of oxidation, is the physiological process by which plants exchange carbon dioxide (CO2) for oxygen (O2). Through this important process, the plant is able to perform photosynthesis. On the other hand, within the types of oxidation, we find fermentation.
Fermentation is a catabolic oxidation process from which an organic compound is obtained as an end product. This final compound is what will dictate what type of fermentation is involved. This can be lactic, alcoholic, butyric, acetic or glycerin.
In living beings fermentation is a fairly common process, since it occurs in microorganisms such as bacteria and also in yeast, as well as in the muscle tissue of animals and humans when the supply of oxygen in the cells is not enough to generate a muscle contraction or to carry out metabolic processes.
Types of oxidation
Slow oxidation
The one that almost always happens in metals due to water or air, causing its corrosion and loss of brightness and other characteristic properties of metals, releasing negligible amounts of heat; when melting a metal, oxidation is accelerated, but the heat comes mainly from the source that melted the metal and not from the chemical process (an exception would be aluminum in autogenous welding).
Fast oxidation
Which occurs during what would already be combustion, releasing appreciable amounts of heat, in the form of fire, and occurs mainly in substances that contain carbon and hydrogen, (Hydrocarbons)
Consequences
In metals, a very important consequence of oxidation is corrosion, a very negative economic impact phenomenon. Combining the oxidation-reduction (redox) reactions in a galvanic cell, the electrochemical cells are obtained (see electric battery). These reactions can be exploited to avoid unwanted corrosion phenomena by means of the sacrificial anode technique and to obtain a continuous electric current.
Oxidation is a pain, especially when it happens with household items that need to be replaced because of it.