CHROMATPGRAPHY; A SEPARATION TECHNIQUE
A separation procedure is a method used to accomplish the removal of different molecules of substances which are alien to each other. At times, these detachments requires filtration method like in the case of refining of bauxite metal for aluminum metal, however a decent case of an inadequate division strategy is oil refining. Raw petroleum happens normally as a blend of different hydrocarbons and polluting influences. The refining procedure parts this blend into different final products, for example:
P.M.S
Kerosene
Gas
Bitumen etc.
However every one of which must be isolated from the crude rough. In both of these cases, a progression of partitions is important to get the coveted final results.
On this note, I will like to introduce you to a separation technique done in the laboratory and it is called chromatography
chromatography
Chromatography is simply defined as the laboratory technique for separating mixtures.Chromatography is a physical strategy for partition in which the segments to be isolated are conveyed between two stages, one of which is (stationary stage) while the other (the versatile stage) moves in an unequivocal bearing.
Types of chromatography
1)Column chromatography: column chromatography is a displacement system in which the stationary bed is inside a tube. The particles of the strong stationary stage or the help covered with a fluid may fill the entire inside volume of the tube (pressed segment) or be focused on or along within tube divider leaving an open, unhindered way for the portable stage in the center piece of the tube (open tubular section).
Planar chromatography: is a division method in which the stationary phase is available as in a plane phase. The plane can be a paper or a wooden material as long as it has a very smooth surface; filling in that capacity or impregnated by a substance as the stationary bed or a layer of strong particles. For example, a glass plate (thin layer chromatography). Distinctive mixes in the example blend fly out various separations as per how emphatically they connect with the stationary stage when contrasted with the portable stage. The particular Retention factor of every compound can be utilized to help in the distinguishing proof of an obscure substance.
2)Paper chromatography: Paper chromatography is a system that includes putting a little line of test arrangement into a portion of chromatography paper. The paper is placed in a container containing a shallow layer of dissolvable solvents and fixed materials. As the dissolvable goes through the paper, it meets the blend which begins to move up the paper with the dissolvable. This paper is made of cellulose, a polar substance, and the mixes inside the blend travel more remote on the basis that they are non-polar. More polar substances bond with the cellulose paper all the more rapidly, and thus, they don't go far.
3)Thin layer chromatography: Thin layer chromatography is generally utilized in research facilities and is like paper chromatography. In any case, rather than utilizing a stationary period of paper, it includes a stationary period of a thin layer of adsorbents like silica gel, alumina, or cellulose. Contrasted with paper, it has the benefit of speedier runs, better partitions. For far better determination and to take into account evaluation, superior Thin Layer Chromatography can be used.

picture of a gas chromatography
4)Gas chromatography: Gas chromatography (GC), additionally once in a while known as Gas-Liquid chromatography, (GLC), is a detachment procedure in which the versatile stage is a gas. Gas chromatography is constantly completed in a section, which is commonly "stuffed" or "narrow".
Gas chromatography (GC) depends on a segment harmony of analytes between a strong stationary stage (regularly a fluid silicone-based material) and a versatile gas (frequently Helium). The stationary stage is clung to within a little distance across glass tube (a slender section) or a strong lattice inside a bigger metal tube (a stuffed segment). It is broadly utilized as a part of investigative science; however the high temperatures utilized as a part of Gas Chromatography makes it unsatisfactory for high atomic weight biopolymers or proteins (heat will denature them), regularly experienced in organic chemistry, it is appropriate for use in the petrochemical, ecological observing and remediation, and modern compound fields. It is additionally utilized broadly in science exploration.
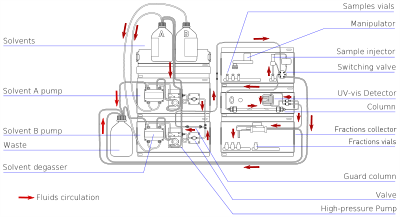
5)Liqiuid chromatography: is a detachment system in which the versatile stage is a fluid. Fluid chromatography can be completed either in a section or a in planar mode. Modern day fluid chromatography uses little pressing particles and a moderately high weight is alluded to as High Performance Fluid Chromatography (HPLC).
In the HPLC strategy, the example is constrained through a segment that is stuffed with sporadically or circularly formed particles, a permeable solid layer (stationary stage) or a permeable film by a fluid (versatile stage) at high weight. HPLC is verifiably isolated into two diverse sub-classes in light of the extremity of the versatile and stationary stages. Strategies in which the stationary stage is more polar than the portable stage (e.g. toluene as the versatile stage, silica as the stationary stage) are named ordinary stage fluid chromatography (NPLC) and the inverse (e.g. water-methanol blend as the versatile stage and C18 = octadecylsilyl as the stationary stage) is named Reversed phase Liquid Chromatography (RPLC).
Terms commonly used in chromatography
1)The analyte is the substance to be isolated amid chromatography.
2)Analytical chromatography is utilized to decide the presence and perhaps at the same time the convergence of analyte(s) in an example.
3)A reinforced stage is a stationary stage that is covalently clung to the help particles or to within mass of the section tubing.
4)The eluate is the portable stage leaving the section.
5)The eluent is the dissolvable that will convey the analyte.
6)A chromatogram is the visual yield of the chromatograph. On account of an ideal division, diverse pinnacles or examples on the chromatogram relate to various segments of the isolated blend.
7)A chromatograph is gear that empowers a modern division e.g. gas chromatographic or fluid chromatographic division.
8)The test is the issue examined in chromatography. It might comprise of a solitary part or it might be a blend of segments. At the point when the example is dealt with over the span of an examination, the stage or the stages containing the analytes of intrigue is/are alluded to as the example though everything out of intrigue isolated from the example previously or throughout the investigation is alluded to as waste.
9)The mobile stage is the stage which moves in a clear bearing. It might be a fluid (LC and CEC), a gas (GC), or a supercritical liquid (supercritical-liquid chromatography, SFC). The portable stage comprises of the example being isolated/examined and the dissolvable that moves the example through the section. On account of HPLC the versatile stage comprises of a non-polar solvents.

Congratulations @impacter! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Do not miss the last post from @steemitboard:
Vote for @Steemitboard as a witness to get one more award and increased upvotes!