THE ATOMIC CRUST
THE ATOMIC CRUST
Orbit
It is a concept that derives from Bohr's atomic theory. It represents the trajectory described by an electron in its turn around the nucleus.
Orbital: It is a concept that derives from the quantum mechanical theory of the atom. Represents the area of space in which there is a probability of finding the electron. Precisely the zone of maximum probability coincides with Bohr's orbit.
Electrons are found at different energy levels within the atom, which are characterized by parameters called quantum numbers, which are parameters that allow us to locate the electron inside the atom.
NÚMERO CUÁNTICO PRINCIPAL.
In the cortex, electrons are placed following certain paths called orbitals. Each orbital is defined by three quantum numbers, which determine the size, shape and orientation of the orbital.
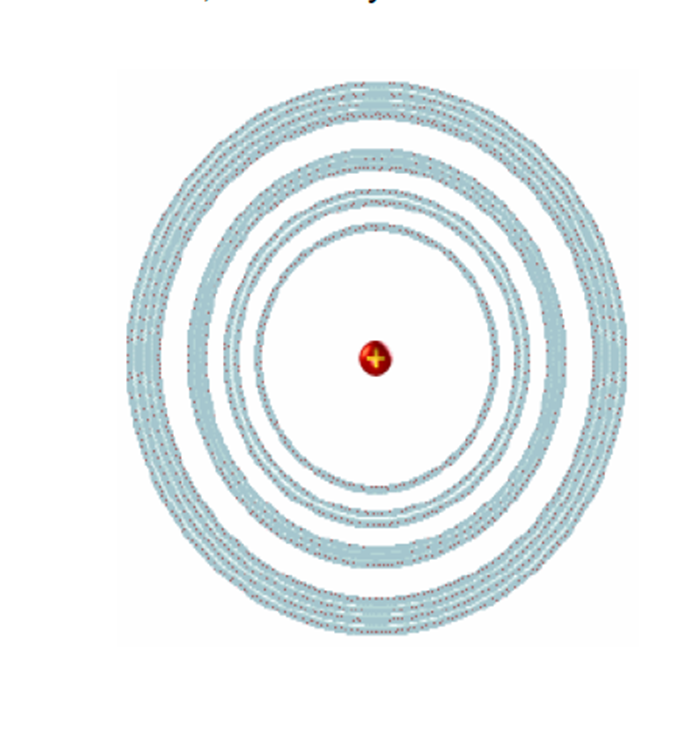
The main quantum number, n, determines the size of the orbital. It can take any natural value other than zero: n = 1, 2, 3, 4 ...
Several orbitals can have the same main quantum number, and indeed they do, grouping in layers. Orbitals that have the same principal quantum number form an electronic layer.
The greater the main quantum number, the greater the size of the orbital and, at the same time, further away from the nucleus will be located.
QUANTUM NUMBER AZIMUTAL.
El número cuántico azimutal, l, indica la forma del orbital, que puede ser circular, si vale 0, o elíptica, si tiene otro valor. El valor del número cuántico azimutal depende del valor del número cuántico principal. Desde 0 a una unidad menos que n. Si el número cuántico principal vale 1, n = 1, el número cuántico azimutal sólo puede valer 0, ya que sus posibles valores van desde 0 hasta una unidad menos que n.
If, on the contrary, the main quantum number is 6, n = 6, the azimuthal quantum number can take six different values, from zero to five: l = 0, 1, 2, 3, 4 or 5
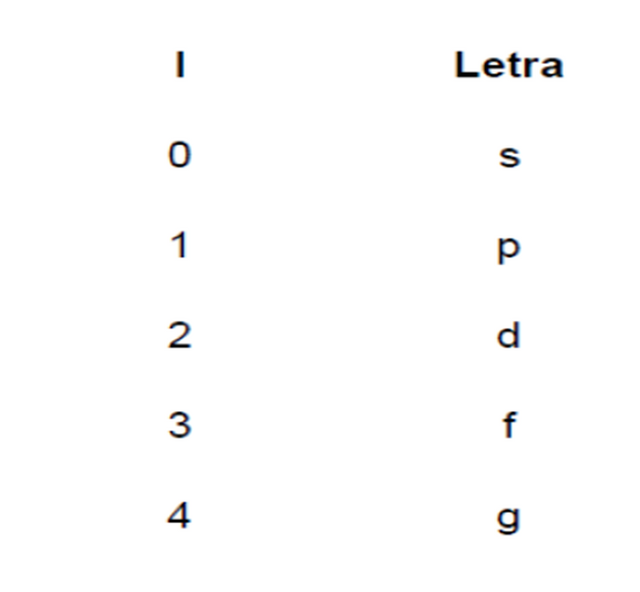
A cada valor del número cuántico azimutal le corresponde una forma de orbital, que se identifica con una letra minúscula:
letter
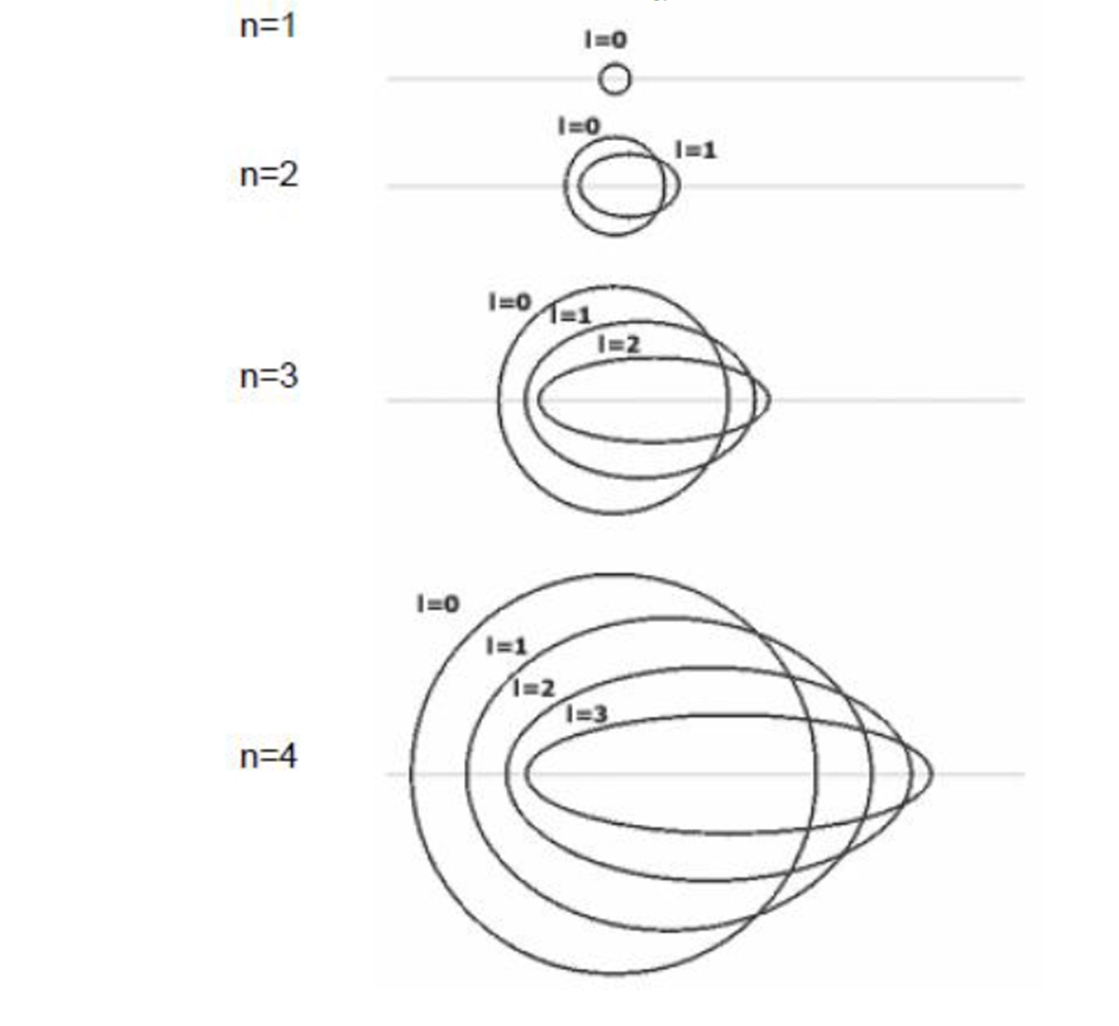
The greater the azimuthal quantum number, the more elliptical and flat the orbital will be. When it is zero, the orbital is circular. When it is worth one, it is somewhat elliptical. If two, it is more flattened; if three, more still ...
QUANTUM MAGNETIC NUMBER
The magnetic quantum number, m, determines the orientation of the orbital. The values you can take depends on the value of the azimuthal quantum number, m, varying from - l to + l.
If the azimuthal quantum number is 0, l = 0, the magnetic quantum number can only take the value 0. Thus, there is only one s orbital. If the azimuthal quantum number is worth 1, l = 1, the magnetic quantum number can take the values -1, 0 and 1, since its possible values go from - l to l. There are, therefore, three p orbitals, since if l = 1 the orbital is called p.
In general, for a value l, there will be 2 · l + 1 orbitale.

good my friends this is the first part of this interesting topic I hope you like it and soon I will be talking more about this topic I hope you like @jesusalbertorios
It is a concept that derives from Bohr's atomic theory. It represents the trajectory described by an electron in its turn around the nucleus.
Orbital: It is a concept that derives from the quantum mechanical theory of the atom. Represents the area of space in which there is a probability of finding the electron. Precisely the zone of maximum probability coincides with Bohr's orbit.
Electrons are found at different energy levels within the atom, which are characterized by parameters called quantum numbers, which are parameters that allow us to locate the electron inside the atom.
In the cortex, electrons are placed following certain paths called orbitals. Each orbital is defined by three quantum numbers, which determine the size, shape and orientation of the orbital.
The main quantum number, n, determines the size of the orbital. It can take any natural value other than zero: n = 1, 2, 3, 4 ...
Several orbitals can have the same main quantum number, and indeed they do, grouping in layers. Orbitals that have the same principal quantum number form an electronic layer.
The greater the main quantum number, the greater the size of the orbital and, at the same time, further away from the nucleus will be located.
El número cuántico azimutal, l, indica la forma del orbital, que puede ser circular, si vale 0, o elíptica, si tiene otro valor. El valor del número cuántico azimutal depende del valor del número cuántico principal. Desde 0 a una unidad menos que n. Si el número cuántico principal vale 1, n = 1, el número cuántico azimutal sólo puede valer 0, ya que sus posibles valores van desde 0 hasta una unidad menos que n.
If, on the contrary, the main quantum number is 6, n = 6, the azimuthal quantum number can take six different values, from zero to five: l = 0, 1, 2, 3, 4 or 5
A cada valor del número cuántico azimutal le corresponde una forma de orbital, que se identifica con una letra minúscula:

The greater the azimuthal quantum number, the more elliptical and flat the orbital will be. When it is zero, the orbital is circular. When it is worth one, it is somewhat elliptical. If two, it is more flattened; if three, more still ...

The magnetic quantum number, m, determines the orientation of the orbital. The values you can take depends on the value of the azimuthal quantum number, m, varying from - l to + l.
If the azimuthal quantum number is 0, l = 0, the magnetic quantum number can only take the value 0. Thus, there is only one s orbital. If the azimuthal quantum number is worth 1, l = 1, the magnetic quantum number can take the values -1, 0 and 1, since its possible values go from - l to l. There are, therefore, three p orbitals, since if l = 1 the orbital is called p.
In general, for a value l, there will be 2 · l + 1 orbitale.

good my friends this is the first part of this interesting topic I hope you like it and soon I will be talking more about this topic I hope you like @jesusalbertorios