How Much Is The Atomic Amount In Sand Granules Compared To Sand Dune
This problem is actually not well defined because the size of the sandbar is not determined. The size of a grain of sand was not mentioned. However, we will see in the end that the number of atoms in a grain of sand will still be more than the amount of sand in the mound.
In chemistry and physics, the Avogadro constant, named after scientist Amedeo Avogadro, is the number of constituent particles, usually atoms or molecules, that are contained in the amount of substance given by one mole. Thus, it is the proportionality factor that relates the molar mass of a substance to the mass of a sample. The Avogadro constant, often designated with the symbol NA or L, has the value 6.022140857(74)×1023 mol−1 in the International System of Units (SI). (The parentheses there represent the degree of uncertainty.).
Avogadro constant: Wikipedia source
We remember in physics and chemistry, one mole of elements were known to contain about 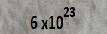 atoms. This number is known as Avogadro's number. The mass of one mole of material is the same number of grams as the mass number.
atoms. This number is known as Avogadro's number. The mass of one mole of material is the same number of grams as the mass number.
Fundamental units may be atoms, molecules, or formula units, depending on the substance concerned. At present, our best estimate of the number of atoms in 12.000 g of 12C is 6.022 x 1023, a huge number of atoms. This is obviously a very important quantity. For historical reasons, it is called Avogadro's Number, and is given the symbol NA.
Mole source
Sand does not contain only one chemical element, but we can assume sand has a certain atomic mass of 30 and a mass density of  . Thus, 1 mole of sand has a mass of 30 grams and a volume of
. Thus, 1 mole of sand has a mass of 30 grams and a volume of 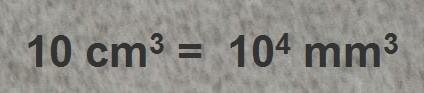 .
.
We can say a cube of sand with ribs 1 mm long, the volume is 1 mm3. Because 1 mole of sand (many grains of sand) has a volume of  ,
,
a grain of sand should contain about
 moles of the atom.
moles of the atom.
So, the number of atoms in a grain of sand is approximately  .
.
which we can round upwards, as many as  atoms. The question is, is the value of
atoms. The question is, is the value of  more than the amount of sand on the mound?
more than the amount of sand on the mound?
Now we imagine how much sand dune happens if  grains of sand are crammed. Earlier it was stated that the assumption of a grain of sand had a volume of
grains of sand are crammed. Earlier it was stated that the assumption of a grain of sand had a volume of .
.
If we multiply by  , the total volume will be
, the total volume will be  . This number is still bigger than the long tube, the rib is 1 kilometre!
. This number is still bigger than the long tube, the rib is 1 kilometre!
When talking about bumps, we might remember childhood playing sand on the beach. At best we see the biggest sandbar as high as our bodies. We certainly never see sand dunes that are long, wide, and 1 km high.
That is why we say that the number of atoms in a grain of sand is still more than the amount of sand in a sandbar that we usually see every day. However, if we replace the "sandbar" in the initial problem with the "desert", the results can be different.
As mentioned,  grains of sand will give a volume of
grains of sand will give a volume of  We can arrange the volume of sand into 10 meters of the sand layer with a square area of 100 km × 100 km. This area is not something extraordinary to be a broad measure of a desert. Thus, it could be that the number of grains of sand in the desert would be more than the number of atoms in a grain of sand.
We can arrange the volume of sand into 10 meters of the sand layer with a square area of 100 km × 100 km. This area is not something extraordinary to be a broad measure of a desert. Thus, it could be that the number of grains of sand in the desert would be more than the number of atoms in a grain of sand.
It should be noted that the approximation method described above is a thought process commonly practised by physicists. To answer a problem that seems cursory at first glance, physicists will simplify it with various assumptions that can approach the truth. We don't need to be fixated on some details, but just make logical assumptions and describe the situation in question.
Reference:
Avogadro constant: Wikipedia source

If you would like to support the educational community by delegating to @steemiteducation, please click on any of the following links. This will ensure that more teachers are supported on a daily basis.
100SP 200SP 300SP 400SP 500SP 750SP 1000SP 2000SP 3000SP 4000SP 5000SP 10,000SP 25,000SP
I upvoted your post.
Keep steeming for a better tomorrow.
@Acknowledgement - God Bless
Posted using https://Steeming.com condenser site.
Thank you