Engineering Slut || How medical treatments have been a source of concern to us as engineers
We live in a dynamic world that is always moving. Nothing will get done if everything stayed still. It is this very movement that gives us life, that allows the blood to flow through our veins and gives actions to our stories.

Source: Pixabay (Public domain - CCO licensed)
But what does that mean to us as engineers? What is really happening when things move? Well, since pretty much everything has mass, most of the movement in our world is associated with mass transfer. It is critical to our lives, our work and something that we should learn more about.
We have discussed a great deal about transfer in our previous posts. Whether it was momentum transfer, as we saw with moving fluids, or heat transfer, like stepping out in the sun or touching a hot pan, we have seen how things move and change. But when people think of mass transfer, they often think of something pretty simple – the movement of an object or fluid’s mass, from one place to another. But that is not really what it is. That is more like bulk fluid movement.
Mass transfer with respect to chemical substances and drug delivery
No, what we mean by mass transfer is a bit more complicated. Mass transfer is the movement of individual molecules and components within a larger mixture or substance. So, it deals with what makes up something like a stream of water rather than its bulk movement as a whole.
Now, because the mass transfer works on this often microscopic scale, it has a lot to do with chemical separation and can literarily be crucial to life itself. To see what I mean, think of drug delivery. And no, I don’t mean like driving around a delivery truck of pharmaceuticals. I’m talking about medicine moving within the body itself.
Medical treatment depends on medicine getting to the right place in your body. It often needs to go through your mouth, to your stomach, and then into your bloodstream. Or maybe it needs to be injected into a certain location. But a drug must be less effective if it’s not taken correctly. Sometimes, it won’t even work at all.
For instance, people with Type 1 and even Type 2 diabetes have to take insulin by injection, in places like their arms or their stomachs. Just swallowing insulin, in pill or liquid form, wouldn’t work, because it would break down in the patient’s stomach acid before it could do its job. But let’s assume a certain medicine is taken correctly. Then its individual molecules and chemical compounds have to move through the fluids in your body to the right location.
There’s a lot more to consider than just that, but as its chore, drug delivery is all about mass transfer. It’s about how one type of chemical moves through other chemicals to get where it needs to go in a mixture.
So how does that mass get transferred?
Well, t needs a driving force. We learned that from momentum transfer, the driving force was a difference in velocity. For heat transfer, it was a difference in temperature. For mass transfer, the driving force is a little more complex.
Let’s get back to Thermodynamics. We said that everything was always trying to reach thermal equilibrium or a balance of temperatures. That is why temperature differences drive heat transfer. For mass transfer, we are still trying to reach equilibrium, but this time, it’s a chemical equilibrium.
Chemical equilibrium
Chemical equilibrium can be explained in terms chemical potential and fugacity, but those are pretty abstract concepts and difficult to directly measure. Which, we as engineers, need to do. So, for our purposes, a better way to explain chemical equilibrium is to approximate it in terms of concentration. Simply put, concentration is how much there is of something in a certain amount of space, or volume.
Concentration
Here, we will look at concentration related to mass, so it will be mass per volume. So, for something to reach chemical equilibrium, it’s going to need to have the same concentration throughout. That means that when left alone, and not acted upon by another force, particles won’t move from one location to another within a fluid or a substance if there isn’t a concentration difference within it. No concentration difference, no mass transfer.

Source: Pixabay (Public domain - CCO licensed)
If there is a difference, then the concentration will move from the higher concentration to the lower one. But concentration isn’t just something we need to worry about for a substance or fluid on its own, but also when it mixes with something else. To keep things simple, let’s just talk about fluids for now.
Let’s also assume that the fluids are miscible, which means that they can be completely mixed together. So when one fluid is put into the other one, we can view the combined fluids as a single mixture in terms of mass transfer. That means that the fluids will mix together until the solution has the same concentration throughout it.
Well, how do they do that?
One major way is through diffusion. Diffusion is when the molecules of two or more things intermingle as they move from higher concentrations to lower ones. To see what I mean, let’s use an example that you can do at home!
If you take a drop of red food coloring and mix it in with a glass of water, you will notice that the red color slowly spreads throughout the glass until the solution is the same color. That is diffusion! And the more food coloring you add, the darker and redder the solution will be. No matter how much you add, it will eventually diffuse into one uniform color, given enough time. And how fast something diffuses can be very important as well. This especially comes into play when thinking about safety.
For example, you will want to know how fast certain acidic or corrosive chemicals can move through your protective rubber gloves. Or how fast chemicals can enter your body when you are exposed to them. Or how quickly they can diffuse through your skin or organs to reach vulnerable parts of your body. That is why whenever you work with hazardous chemicals, you protect yourself so that the rate of diffusion is as low as it can be.
Catalytic converter
What if you are trying to produce a chemical for sale – or may be just convert a toxic chemical into something safer so you can pass the raw material through a catalytic converter. Reactions will certainly occur on the surfaces of all the catalytic particles inside, but how fast the molecules diffuse to the catalyst surfaces will affect how much product you get, and how fast the reaction will even occur.
Fick’s law
Now, if we really want to be able to understand and quantify mass transfer, then we are going to need to learn about Fick’s law.
Fick’s law that there is a drift, or movement, of particles from denser regions to ones that are less dense, or that they are moving from a high concentration to a lower one.
So, pretty much we just learned. Fick’s law defines the rate of this drift – the diffusion, or mass flux in our case, as proportional to the product of the thermophysical property which is the diffusivity constant or coefficient and the driving force – which will be our concentration gradient.

The negative sign here shows us that the diffusion will occur in the direction opposite that of the increasing concentration or from a higher concentration to a lesser one. Now, D will either be called diffusion coefficient, depending on who you ask. But in all practicality, they mean the same thing. That is a debate more of semantics than application.
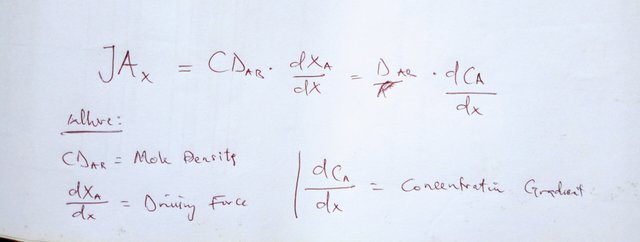
And the concentration gradient you will see here is the derivative of our concentration in the x direction. If you are not familiar with derivatives, they are basically rates of change. What is so important to remember is that, while we have been focusing on the mass transfer particles themselves moving, this movement is going to be making the overall mass of whatever the particles are in move as well. So this small movement can lead to bigger, bulk movement.
Departing thoughts.
The tiny particles won’t be able to get their overall mass to move in certain obstructed directions, like getting water to move through the glass that it’s in, but they can cause bulk motion into more open directions, like the air above the water. In the end, when things are moving, there is a lot going on than just mass transfer. But it does give us the basis to understand some of what is going on. Just because particles are small, don’t count them out. Get enough of them moving and you can make a big change. Now that I have said that out loud, that actually sounds pretty inspirational.
References
- Chemical equilibrium
- Mass transfer
- Catalytic converter
- Concentration
- Diffusion
- Fick's law pf diffusion
This post has been upvoted by the user-run curation platform CI! In this platform users are able to manually curate content. This is done regardless of Steem Power, for both rewards and vote size calculation.
Join in at our site here!
https://collectiveintelligence.red/
Or join us on discord to interact with the community!
https://discord.gg/sx6dYxt
This post was submitted for curation by: @gentleshaid
This post was given a rating of: -0.3505783627497891
This post was voted: 0%
Congratulations @kafilat! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!