The Impressed Current Cathodic Protection System; giving corrosion a dose of its own medicine
Because of the fact that the type of environment has a part to play in the corrosion and some environments are more corrosive than some other ones, the rate or even the possibility of corrosion depends on the environment amongst other factors. However, the case of corrosion is more common when it comes to moist or wet environments especially when the metal in question is ferrous or an alloy of Iron (Fe).

Well, we should know what caused the corrosion of this vessel and as you can see, the paint couldnt stop it.source: Wikipedia commons under the Creative Commons Attribution-Share Alike 2.5 Generic, 2.0 Generic and 1.0 Generic license.
In my previous articles, I highlighted the various forms by which corrosion can occur and the methods by which corrosion can be combatted. In the latter article, I mentioned the cathodic protection method as made of two types but I mentioned only one – the galvanic anode cathodic protection method. Today, we would be discussing the second method whose name I would be mentioning later. Stay tuned.
In order to win a fight (kung-fu, wrestling or even boxing) some coaches would train their fighters and encourage them to use the opponent’s strength against them. I watched a movie that had Jackie Chan playing the lead role (I can’t remember the title) and I can remember one scene where he was faced with a hefty man about twice his body size. He fought and according to him, he defeated the man by simply “using his strength against him”. I cannot really explain how that went because there was a lot of stunts involved and this is not a movie premiere.
You might be wondering how this relates to corrosion, well, here is the answer. The cathodic protection method (as highlighted in the previous article) shields a metal from corrosion by making it the cathode in the corrosion cell. That means, the corrosion cell is still complete and the corrosion process is still occurring but not at the detriment of the metal of interest – a case of using the opponent’s strength against him (or it). So, instead of the material of interest to supply the electrons that flow through the cell and corrode, the electrons are introduced form another source. Is that not more of like using the power of corrosion against itself?
In the galvanic method, the sacrificial anode supplies these electrons that flow through the corrosion cell and gets used up in the process and thus there is always the need to check and change anodes that have been used up.
This presents a major disadvantage for the galvanic method because in cases where the electrons required to do the job (in terms of current) is high, the galvanic anode gets used up pretty fast and if care is not taken, the metal of interest might get corroded as well. Also like I mentioned earlier, environments such as those that have a higher resistivity can reduce the effectiveness of the system. Also, when used for water vessels such as ships, attaching galvanic anodes tend to increase the weight of the vessel and thus affect drag.
With all these disadvantages and more concerning the galvanic anode system, you would agree with me that there is the need for a system that can deliver the protection required more efficiently. That system is the impressed current cathodic protection system.
What then is the impressed current cathodic protection system?
In order to transport fluids such as petroleum, gas and the likes, pipelines are used and they usually run through miles underground. These metallic pipelines in the ground are susceptible to galvanic corrosion and if not managed properly would result in loss of the product, pollution of the environment and possible loss of life and property.
Using corrosion protection methods such as coating would not be able to completely protect the pipeline from corrosion as the coating would fade off with time and expose the pipeline to corrosion. I don’t think I need to once again state the effects that corrosion would cause for the pipeline. just like in the image beside, loss of the products in the pipeline is imminent all thanks to the corroding metal. This also applies to ship hulls, water tanks,underground storage systems or concrete reinforcement (yes, the steel rods that are used to reinforce concrete also corrode and can cause structure failure).
Impressed current cathodic protection system is the type of cathodic protection system in which the current required to make the material of interest a cathode in the corrosion cell is supplied from an external source. Just as the name implies, the current is impressed. The current is usually delivered from a DC source.
It is used in situations where the current required for the corrosion protection of a material is more than can be obtained by applying the galvanic system for protection (yes, the required current for protection can be calculated). Calculating the current required for protection can be calculated given the following parameters; the surface area of the material to be protected, the current density of the environment and the efficiency of the coating used. I wouldn’t want to bother you with the boring calculations, so let us move on.
Just like the galvanic system, the impressed current system also aims to complete the corrosion cell while making sure the material of interest is the cathode. However in contrast to the galvanic system, an external source supplies the current even though an anode is present.
How is the impressed current applied?
The impressed current cathodic protection system resembles the electrolytic cell as there is a source of electricity present in the system. The power source however being a DC source.
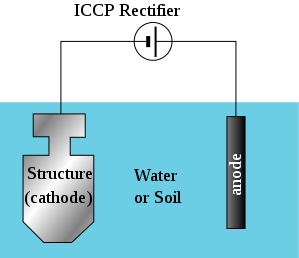
In the impressed current Cathodic Protection System, the positive terminal of the DC source is connected to the piece of metal that is to serve as the anode while the negative terminal is connected to the material to be protected. As a result of the presence of the two metals in the electrolyte (soil or water), the corrosion cell is complete and the material of interest is protected as a result of its position as the cathode in the cell.
Unlike the galvanic system where the choice of anode is dependent on the galvanic series (just as I noted in my previous post), the anode can be made of any material depending on the underlying conditions such as the cost, current density and other factors.
I expect you to know that the type of electric current delivered by a national grid is the alternating current and if this is to be used as the source of electric power for the impressed current system, a rectifier (a device that is used to convert Alternating current (AC) to Direct Current (DC)) is used as part of the system. However in regions where there is no access to electric grid, alternative power sources such as solar, wind and the likes can be employed. These sources would deliver electricity to an energy bank (battery) which quite coincidentally (or not) delivers Direct Current (DC). The Impressed current system makes use voltmeters and ohmmeters to measure and monitor the current and voltage of the system. All this is in a bid to ensure the right amount of current is delivered to the material to be protected.
Simply put; the positive and negative terminal of a DC source is connected to the anode and the material to be protected respectively which are present in the electrolyte. As a result of this, the corrosion cell is complete and the material of interest is protected because it acts as the cathode. Kapish!
How much is this system better?
The impressed current cathodic protection system finds application in large scale industries such as in pipelines, large ships, underground storage tanks and the likes. The reliability of this system is what makes it the most suitable form of corrosion protection for large scale applications. Combining this system with other methods such as coating can ensure the longitivity of the lifesapan of the metallic material and thus save a lot of money in the long run.
Unlike its twin, the anodes used in the cathodic protection system lasts longer than the galvanic system (those ones need to be changed periodically) because they are not actively delivering the current needed in the corrosion cell but only serving as a means of conveying this current.
Also, the impressed current system presents the possibility of applying higher values of current in contrast to that of the galvanic system. This way, environments that are of higher resistivity (which would be a big hurdle for the galvanic system) can be comfortably tackled and the material protected from corrosion.
Another way the impressed current system is more efficient than the galvanic system is the fact that the current output can be controlled. This eliminates the possibility of the galvanic system supplying too much or too less current required for corrosion protection. The electrical arrangement of the impressed current system allows for varying the current output in the event of too much or too little current supplied. As a result of this, the advantage of easy monitoring in contrast to the galvanic system which is quite complicated to monitor based on the fact that the current supplied is at the mercy of the anode used.
Well, anything that has advantages will surely have disadvantages. The operation and maintenance of the impressed current system is quite sophisticated and requires a high level of expertise. Also, the presence of so much current in the soil or water poses a problem for metlallic materials in the vicinity as they are at risk of stray currents. As a result of stray currents, the metals in the vicinity would corrode faster that they naturally would.
CONCLUSION
The menace of corrosion has been curbed efficiently over the years all thanks ro the impressed current cathodic protection system. This system simply takes over from the galvanic system and offers more current in cases where the current required for protection is high. By impressing the current form an external DC source, the material is sure to be immune to corrosion (as long as the system is active).
Do you think I am right by saying that this system is the boss of them all and really offers a cool way of combating corrosion?
Although all other methods of corrosion protection are quite efficient in their spheres, the impressed current system does quite a lot based on the sectors they are used for.
REFERENCES
How does ICCP system work?
Impressed current cathodic protection ICCP
Impressed current cathodic protection ICCP system
Cathodic protection
The principle of cathodic protection
Cathodic protection 101
Mars, G. F. (1987). Corrosion Engineering (Third Edition). McGraw-Hill Book Company.



I remember watching you guys carry out something like this.. It was confusing then but very clear now.. good job.
haha, those times were the learning times. thanks for dropping by
Well done @rharphelle! You successfully guessed the match result.
Click on the badge to view your Board of Honor.
Do not miss the last post from @steemitboard!
Do you like the SteemitBoard Corld Cup Contest?
Vote for @steemitboard as a witness and get one more award!
I think this describes very well the electronic rust inhibitor that keeps my old Honda from rusting out. Now I know how it works in so much more detail.
Thanks for dropping by
You said it is the best way in corrosion protection. Nice to understand the rudiments.
thanks for dropping by
You are welcome bro
Hi @rharphelle!
Your post was upvoted by utopian.io in cooperation with steemstem - supporting knowledge, innovation and technological advancement on the Steem Blockchain.
Contribute to Open Source with utopian.io
Learn how to contribute on our website and join the new open source economy.
Want to chat? Join the Utopian Community on Discord https://discord.gg/h52nFrV
Hello @rharphelle
Certainly a well detailed piece! New thing learnt — What then is the impressed current cathodic protection system. I learnt the coating method of protecting metals against corrosion in high school chemistry and physics, but this impressed current cathodic protection system is a total new idea to me. Thanks for taking your time to put this together.
@eurogee of @euronation and @steemstem communities
I am glad i could pass knowledge across. Thanks for dropping by
Well done @rharphelle! You successfully guessed the match result.
Click on the badge to view your Board of Honor.
Do not miss the last post from @steemitboard!
Do you like the SteemitBoard Corld Cup Contest?
Vote for @steemitboard as a witness and get one more award!
Nice one here. I can see why my company during IT made a whole lot of gains.
The post is superb!
Job well done... Superb outlining
I would say you are right
Corrosion sure is the enemy to the this man names iron, and i beleive if it were a human , it will obviously thank the inventor or developer of corrosion inhibition.
Evem the galvanic system is not as effective as the impressed current electrode, reason being that i have see galvanised metals still rust
Nice one here @rharphelle