Stopping Post Surgical Infections With Small Pieces of Protein (Peptides)
I'm back, it's a whole new year, and as such it's about time I assemble a piece for all of you. With that in mind I think I've got something cooked up which some of you may find interesting! Today's discussion will revolve around an article published in Nature Scientific Reports titled "Elimination of Antibiotic Resistant Surgical Implant Biofilms Using an Engineered Cationic Amphipathic Peptide WLBU2."
I know, I know, that title is pretty full of sciency sounding words, which may seem intimidating to some of you. Rest assured, we will break all that down! Lets begin:

Surgery
Before we dive into the article, lets first take a moment to chat about surgery, something that many of us have had or for those who haven't, likely have will at some point in your life. We all know that surgeries carry with them a risk of complications, and some of those complications may even be life threatening. One such complication that can occur even after a successful surgery is an infection. [2] These infections are typically treated with antibiotics which are administered based upon what type of bacterial agent has started to make itself a home.
For some surgical procedures (knee replacements are discussed in the main article) treatment success for these post operative infections isn't all that great, with upwards of one quarter of them leading to death of the patient, despite the initial surgery being a success! [3] If that number is surprising to you, that's because it should be! Why on earth are so many people dying from infections, even when being treated by antibiotics?
Biofilms, Bacterial Persisters and Why Antibiotics Don't Work
To better answer that question we must first talk about two things, biofilms and how antibiotics work in the first place. Lets start with biofilms.

Biofilms (yes that's the fifth time that word appears in this article with no explanation of what it is) are groups of bacteria all clumped together contained within some goopy (ehhh, not a science term) junk called an extracellular polymeric substance. That, is mostly a bunch of sugar and protein molecules which protect the bacteria and keep them attached to where ever they are. One thing to make note of about the bacteria that are living in a biofilm is that many are not metabolically active, they are technically alive, but not dividing, replicating DNA or really doing much of anything that one would think of as "being alive." [4]
Onto our second point of contention, how does an antibiotic work? Simply, antibiotics are just compounds which interfere with the metabolism/growth of bacteria. They do things like, stop the bacteria from making new cell wall materials (this is how penicillin, or penicillin derivative antibiotics work) [5], or stop DNA replication (this is what rifamycin does), still others stop new proteins from being made (streptomycin works like this). [6] If a bacteria can't do these things they die.
...except didn't I mention above that some of the bacteria in the biofilms are not doing any of these things? What happens then, to bacteria which aren't doing what antibiotics stop... do they die as well?
Nope.
And it is because of this that these bacteria are called persisters, because they persist on "living" even in the presence of antibiotics. Now these metabolically inactive but still alive bacteria can become active again, and if they do so after the antibiotic has long been metabolized away by our bodies, they can grow and cause an infection (crap!) This is what happens to so many patients who get implants (knees, etc...), and still die of an infection despite antibiotic treatment. They have a whole bunch of biofilms on those implants composed of bacterial persisters.
Onto The Article!
So now you have some idea why an alternative to antibiotic treatment might be a good idea! We want to stop these bacteria who aren't metabolically active (and the ones who are too). One way to do that is through the use of small protein pieces (peptides) which have antimicrobial activity. Our body naively uses these sorts of peptides as a part of our innate immune system. In the article we are discussing the authors were using a cationic amphipathic peptide (cationic meaning it has a positive charge, amphipathic meaning it has both water loving and water hating parts) called WLBU2 (another beautiful name, bravo fellow scientists, BRAVO!!! would you pronounce that one wahl-boo-too? :D ).
WLBU2 is a man made peptide, which was designed to be very very specific to a bacteria's membrane, but not at all specific to a human cell's membrane. When WLBU2 binds to a bacteria's outer membrane it breaks it a part (it goes for it because the bacterial membrane has a lot of negatively charged spots, and as we discussed above WLBU2 is positively charged.) It's effectiveness against bacteria has been shown in a variety of studies. [7], [8], [9]. Additionally, it has shown good efficacy against antibiotic resistant bacteria in animal models as well. [10]
It had never been tested against these metabolically inactive biofilm bacteria though, and so that is what the authors here set out to do:
What Did They Observe?
Both An Antibiotic and WLBU2 Can Kill Cultures of Staphylococcus aureus
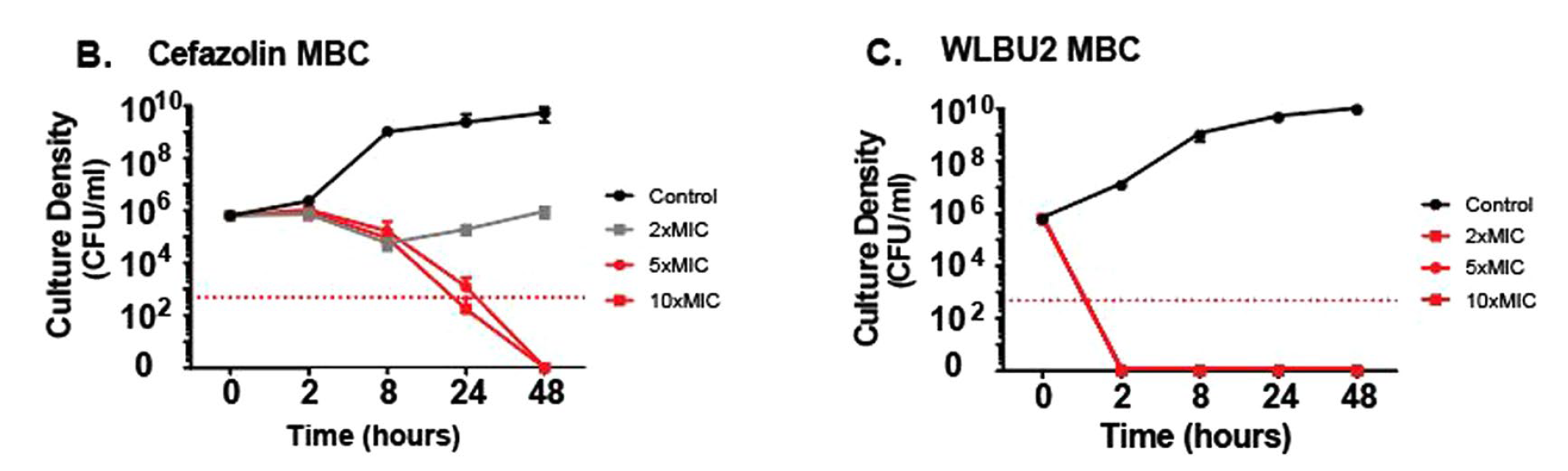
Okay so in this first plot set, we are looking at the amount of growing cells (colony forming units) in a culture of some S. aureus and the amount of time it takes for these numbers to decrease. In the legend on the right you see 2xMIC 5xMIC and 10xMIC... this just means that the amount of the antibiotic, or peptide was 2, 5 or 10 fold more than the Minimum Inhibitory Concentration (or the lowest amount that still kills the cells). We are looking at the antibiotic cefazolin (it works like penicillin) on the left, and the WLBU2 peptide on the right. We can see that both are able to kill the bacteria within 48 hours. Nice.
WLBU2 Kills Biofilm Bacteria Where An Antibiotic Fails
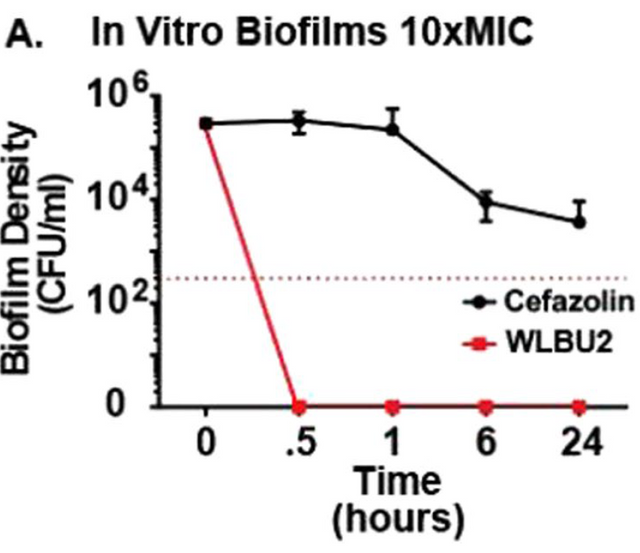
However the story changes when we look at the ability to kill the S. aureus bacteria living in a biofilm. Here they used 10 times the minimum inhibitory dose of the antibiotic and the peptide (WLBU2) and we see that the antibiotic doesn't do much damage while the peptide kicks those bacteria where it hurts (I mean it kills them). Great! But is the WLBU2 really using a different mechanism, or is it still based on the metabolism like the antibiotic would be? This is what they wanted to see next (it should be independent of metabolism... but is it?)
Shuttin Down That Metabolism
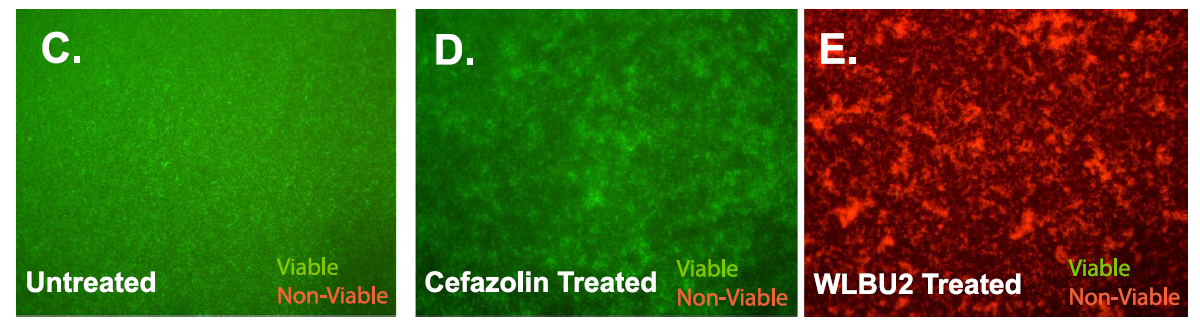
Okay, so here the researchers were using a LIVE/DEAD fluorescence dye kit where cells will glow green if they have an intact membrane and are alive, or glow red if their membrane is broken up and they are dying/dead. Now we already know that under normal conditions the cefazolin antibiotic stops the synthesis of the bacteria's membrane (thats how penicillin (and cefazolin as its related to penicillin) works remember) but this requires metabolic activity. They wanted to check for cell death, even in the absence of metabolism. To do this they treated the cells with carbonylcyanide-m-chlorophenylhydrazone which is a compound that inhibits electron movements in metaboic pathways (or in laymans terms, it shuts down that bacterial metabolism).
What we can see is that un-treated cells are okay, they glow green (left). If the metabolically shut down cells are treated with the antibiotic, they too are okay (green as grass... well not really grass... but green). However, the metabolically challenged cells treated with the WLBU2 peptide, they are dead, as seen by how red they glow in this fluorescence test! So the WLBU2 is killing those cells, even when they are metabolically non functional. This shows that the peptide is indeed using a different mechanism then the antibiotic does to kill the cells. Cool!
Brief Conclusions
WLBU2 has good efficacy against bacteria in these tests, and even more importantly is able to kill the bacteria that are both metabolically inactive and living in a biofilm. It shows at least based on this study, great promise for potential applications in post surgical infections, especially those caused by bacteria known to form these antibiotic resistant biofilms.
Sources
Image Sources
Text Sources
- https://www.nature.com/articles/s41598-017-17780-6
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2812878/
- https://www.ncbi.nlm.nih.gov/pubmed/24352771
- https://www.ncbi.nlm.nih.gov/pubmed/18453274
- http://www.life.umd.edu/classroom/bsci424/Chemotherapy/AntibioticMechanisms.htm
- http://onlinelibrary.wiley.com/doi/10.1002/anie.196806931/abstract
- https://www.ncbi.nlm.nih.gov/pubmed/16048927
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1855554/
- https://www.ncbi.nlm.nih.gov/pubmed/21920706
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3584960/
SteemSTEM
If you haven't heard about the SteemSTEM project yet (WHAT do you live under a rock!? Just kidding), and are reading this post then I highly recommend you take a look into it! The SteemSTEM team has been working for over one year now to promote promote well written/informative Science Technology Engineering and Mathematics postings on Steemit. The project (@steemstem) seeks to build a community of science and technology lovers on steemit and ade in nurturing the growth of blogs that will make steemit a go-to source for science/tech information, news, and just generally fascinating content.
To learn more about the project please join us on steemit.chat (https://steemit.chat/channel/steemSTEM) or on discord, we are always looking for people who want to help in our quest to increase the quality of STEM (and health) posts on our rapidly growing platform!
Finally thanks to @rocking-dave for the great gif set that so many members of the community use in their posts. They look fantastic.
Is there any reason why the health practitioners are not administering a combo of the WLBU2 peptide, and the standard penicillin antibiotics? It is always sad to see someone die as a result of infection after a successful surgery.
There are a lot of problems at the moment. They do aggregate, are chemically and physically unstable, have short half-lives in plasma. While their antibiotic activity is top notch and is improving, now is full of engineering problems.
Fosgerau, K., & Hoffmann, T. (2015). Peptide therapeutics: current status and future directions. Drug Discovery Today, 20(1), 122–128. https://doi.org/10.1016/j.drudis.2014.10.003
Engineering problem is something we would always have. Thanks for the link.
I don't think the peptide has gone through human clinical trials yet.
Ok. That is reasonable then, my primary fear in going under the knife is the post-op complications.
Glad to antimicrobial peptide research on here as I used to study resistance mechanisms to them. I'm guessing they don't know the exact mechanism of action of WLBU2. It usually involves some lipid II binding, but that wouldn't explain why it kills inactive bacteria. Great post!
It kills all bacteria, inactive or or active. You studied this sort of thing and dont know the MOA for this peptide! For shame! (kidding)
I don't know precisely how this particular peptide works, though as it is highly positively charged, it is plausible that it works purely through direct disruption of membrane integrity.
Out of curiosity, what if bacteria becomes resistant to antibiotics? Would it become a serious international problem? And how can we slow down this process (since bacteria evolves and will try to fight antibiotics) ? (cheers from a newbie in biology :D)
There are multiple types and variations of antibiotics. It's almost a competition between finding new antibiotics and bacteria becoming resistant. Bacteria with resistance often pop up in high-usage areas, such as hospitals. Luckily these hospitals are often equipped well for isolation of the bacteria so that it doesn't spread. So it really depends on a lot of factors.
One of the best tools we have in combatting antimicrobial resistance is not, in fact, creating new antibiotics. But in better controlling and monitoring how we use the antibiotics we have, often called Antimicrobial Stewardship. If we limit the amount of exposure that bacteria have to antibiotics then there won't be a strong enough selective pressure for them to evolve the resistance we are all so afraid of!
We should always be doing that though. We should also always be developing new antibiotics.
There are already antibiotic resistant bacteria. Have you ever heard of MRSA? That is a bacteria (its actually a S. aureus bacterial strain, similar to what was used in the study this post is about) which is resistant to basically all of the antibiotics that we use (there are a few last ditch ones which may work). Well it is a problem already and there are a few working antibiotics left. How can we slow the process? We can't, but we can work to identify new antibiotics, or other ways to kill the bacteria (maybe something as crazy as nano-robots will be the solution far in the future!)
Many thanks to both of you for telling me this! The future looks grim with these super strong bacterias.. Let's hope there's going to be a breakthrough in the near future to make sure these bacterias won't spread around like wildfire.
Well there are breakthroughs... like the one this post is about!
You actually explained this lengthy title pretty well.
But how can bacteria become active or inactive? Can they move from an active state to an inactive state at will?
I do not know the mechanism by which cells enter the persister state. However I do know that it is something like 1% of the cells in a biofilm are in this state.
One mechanism I studied about is that some bacteria, like bacillus and clostridium species have the ability to form spores when they are in a harsh or difficult environment. These spores are much more resistant to damage like heat, acid, alkali than the bacteria themselves. The spores are their dormant state, also known as their inactive state. When the environment is favorable again, the spores can germinate back into the active form.
Thanks for providing some answers :)
My pleasure, sir. :)
Nicely done here, and very interesting article. Antibiotic resistance is a huge problem as many of the bacteria we are faced with are constantly mutating and becoming more and more difficult to treat... this is where health literacy and health education becomes very important, such that we take all the necessary measures to prevent this continuing rise of antibiotic resistance ( I think doctors over prescribing medications is honestly one of the biggest factors that plays into this ) as well as patients not finishing there entire script because they think the infection is already gone because they are no longer experiencing symptoms... thus health literacy surrounding this topic area must be fully emphasized from patients all the up to our top performing physicians!!
WLBU2 seems to have a promising future, and could definitely be a go to alternative in the face of this problem... awesome stuff @justtryme90 really enjoyed your steemstem article!!
Respect from @conradsuperb
Thank you very much @contradsuperb! I am glad you enjoyed the post, and you are very correct about the need for additional knowledge about upcoming alternatives for physicians. Antibiotic resistance will be a big problem in the coming decades, we need to be prepared now with methods to deal with it.
Very informative article. It's good to know that we have WLBU2 now ( can't they think of a more normal sounding name? Scientist and their non pronounceable naming sense... ) . Anyway this research will surely save more lives. As many bacteria are immune or gaining immunity to antibiotics especially penicillin. Thanks and have a nice day.
I make fun of it in each of my posts!
If it became a drug it would be given a more normal name.
Thanks for reading!
Thanks for the reply. I hope they can make a drug soon, as we won't know when will be needing to undergo surgery. I will feel more secure knowing that, I will be experiencing less complications after surgery. Thanks again.
I'm an Italian resident in Urology and I really appreciated this article, very well written.
I didn't get if the WLBU2 peptide will have a topic role or systemic.
Can I have your opinion?
I don't think that is known (what the intentions for use would be say in a clinical trial), but based on the location of the infections (and biofilms on a surgical implant) would likely be administered locally. However they discuss it's lack of effect on eukaryotic cells, so that doesn't rule out the possibility for systemic use.
well I completly agree with this point of view. I was thinking about the possible role of WlBU2 in penile prosthesis "coated" with it, and maybe ( if the price its affordable) the possibility to use it in a permanent catheterization
Id say its a little ways off from those sorts of considerations at this time.
This is certainly a very interesting approach. But it still has the same risk we're facing with antibiotics today, development of resistance. It is after all a protein that will target some sort of bacterial antigen. And if we have learned anything from the mistakes of the past, it is that these bacteria are super smart and they will mutate and develop resistance at some point in time.
I think one way to fight this would be to do what we are trying now with antibiotics : Using a combination of antibiotics to prevent development of resistance. It took us years to realize this strategy with antibiotics by which time lot of our great antibiotics like were already rendered useless by the bacteria.
But now that we already have this strategy, I think it would be very wise to implement the same here with these antimicrobial peptides. What i think would be a wise step is to wait a little longer before starting to use WLBU2 already and maybe try and engineer a few more of these peptides, each targeting different antigens on the bacterial membranes and then declare war on the bacteria!!!
Would love to hear whether you agree on it or if you think this might not yet be possible.
Not necessarily. Its mechanism is very different from how antibiotics work it would seem.
No, but micro-evolution is fast!
By your above logic that won't matter, just as combination antibiotic therapies don't stop the development of resistances.
I am not 100% certain how the cationic peptide works, but it would appear somewhat nonspecifically based on surface charge. As such I don't think the bacteria can evolve that away quickly.
Thank you for the corrections :)
I wasn't really correcting just chatting :)
Hahaha but I learnt something new!!! Always great chatting with more learned people!!
Cheers!!
We'll explained! Thank you very much for that.
There's just one thing I'd like to add: it may look promising in vitro, but that doesn't mean it's going to work in vivo. Looking forward to further studies (and explanations thereof)!
In vivo studies have already been done on this peptide as far as efficacy against bacterial infection (just no human clinical trial, mouse model was tested), they are mentioned in the post (and cited). Studies looking at the in vivo efficacy with regards to this specific biofilm fighting property however have not been done.
Glad you liked the post, have a great one.
Very informative post with much of it way beyond my understanding but when in the hospital I am always afraid of staphy infections. Anything that will help eradicate that is great news.
Is there anything that I could do to help you understand it better?
Thanks, I just need to re-read and concentrate on what I am reading. Thanks for the offer of help!